


|
|
To see this report in Portable Document
Format (PDF), please click on the button. ![]() Should you not have Adobe
Acrobat Reader (required to read PDF files), this free program
is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Should you not have Adobe
Acrobat Reader (required to read PDF files), this free program
is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Open Forum: In an attempt to promote free and open discussion of issues, The Agrichemical and Environmental News encourages letters and articles with differing views. To discuss submission of an article, please contact Dr. Allan Felsot at 509-372-7365 or afelsot@tricity.wsu.edu; or Dr. Catherine Daniels at 509-372-7495 or cdaniels@tricity.wsu.edu. The newsletter is available in a hardcopy version for a $15 yearly subscription fee. Please contact newsletter editor Sally O'Neal Coates at 509-372-7378 or scoates@tricity.wsu.edu for details.
This page has been accessed times since December 22, 1999.
A research report recently published in the peer-reviewed, scientific journal Veterinary and Human Toxicology (Vol. 41, No. 2, 1999) raises some very troubling questions about an earlier National Cancer Institute (NCI) study on the use of the herbicide 2,4-D and the incidence of canine malignant lymphoma (CML), a form of cancer peculiar to dogs. The NCI study published in the Journal of the National Cancer Institute (Hayes et al., 83:1226-31, 1991) purportedly showed not only a significant association between the use of 2,4-D on lawns and the incidence of CML among pet or companion dogs, but a significant dose response as well. In other words, the more often you treat your lawn with 2,4-D, according to the NCI study, the higher the risk of cancer to your dog.
These findings are contrary to the extensive
toxicology of 2,4-D animal feeding studies conducted under EPA/Good
Laboratory Practices. Nevertheless, the NCI study received extensive
coverage in the media, being carried by all major news services
(AP, Reuters, New York Times News Service, etc.) and brought renewed
cries from advocacy groups for banning 2,4-D.
The current report, however, failed to show either a significant association between 2,4-D and cancer or the dose response, the widely publicized findings in the NCI study. What was unusual about the most recent report was that it used the same raw data developed by the National Cancer Institute in the original NCI study. This information was obtained, over the NCI author's objections, through the Freedom of Information Act (FOIA) by the 2,4-D Task Force. The data package received from the National Cancer Institute was then turned over to Dr. John Kaneene, Director, Population Medicine Center, School of Veterinary Medicine, Michigan State University, for independent analysis.
In December 1996, Dr. Kaneene submitted a detailed report of his findings to NCI's Dr. Hayes, giving him the courtesy of a response prior to the publication of Dr. Kaneene's paper. To date, Dr. Hayes has chosen not to respond. In other words, he has apparently chosen not to defend his highly publicized study. Just how the NCI study concluded that there was a significant association between 2,4-D use and canine cancer remains something of a mystery. The FOIA request specifically asked to see the algorithm NCI used to determine the alleged dose response, something that NCI has never provided.
What in the world is going on here?
 Why would a scientist publish conclusions that apparently
cannot be supported by his own raw data? What would such a scientist
gain? The publication of any study showing an association between
a major pesticide and cancer or other serious health problem invariably
guarantees the author extensive media attention, invitations to
speak to various groups, etc. Perhaps more importantly, it can
also stimulate new sources of funding or grant money. Only rarely
does anyone bother to examine the researcher's raw data. That
sort of thing is just not covered in the usual peer review process.
Why would a scientist publish conclusions that apparently
cannot be supported by his own raw data? What would such a scientist
gain? The publication of any study showing an association between
a major pesticide and cancer or other serious health problem invariably
guarantees the author extensive media attention, invitations to
speak to various groups, etc. Perhaps more importantly, it can
also stimulate new sources of funding or grant money. Only rarely
does anyone bother to examine the researcher's raw data. That
sort of thing is just not covered in the usual peer review process.
But this story goes beyond the canine lymphoma study. It begins back in 1986. The publication of the so-called Kansas Farmworker Study (Hoar et al., JAMA, 256(9):1141-7) showed a significant association between "Non-Hodgkins Lymphoma (NHL) in Relation to Duration, Frequency, and Latency of 2,4-Dichlorophenoxyacetic Acid (2,4-D) Use" (ED. NOTE: their Table 3 is reproduced below). The study seemed to confirm at least the NHL portion of earlier Swedish studies which suggested an association between phenoxy herbicide use and the incidence of both NHL and soft-tissue sarcoma (STS). Other studies, including the Kansas study, have failed to support the reported association between phenoxy herbicides and STS.
The Kansas study was even more widely publicized than the dog study, receiving extensive print, radio and television coverage. Advocacy groups began calling on the Environmental Protection Agency (EPA) to ban 2,4-D, and EPA did place 2,4-D in pre-Special Review status. A major national lawn care company stopped using 2,4-D as a result of the publicity.
 When others noticed that the questionnaire used
in the Kansas study asked for information on general herbicide
use only, and did not develop any data specific to 2,4-D, questions
were raised about how the associations reported in Table 3 were
supported. The author responded by issuing a correction (see Correction,
JAMA, Vol. 256, No. 24, 1986), changing the name of the table
to "Non-Hodgkin's Lymphoma in Relation to Herbicide Use .
. ." rather than 2,4-D use. However, the already extensively
publicized study was by then widely perceived as a 2,4-D study,
and it still is. How could such a significant error have occurred?
Was it simply an honest mistake? In the numerous times that NCI
has cited the Kansas study since, this writer has yet to see one
instance where NCI also cites the important correction. It seems
as if the National Cancer Institute wants the public to believe
that the Kansas study, as published, was a "2,4-D study."
When others noticed that the questionnaire used
in the Kansas study asked for information on general herbicide
use only, and did not develop any data specific to 2,4-D, questions
were raised about how the associations reported in Table 3 were
supported. The author responded by issuing a correction (see Correction,
JAMA, Vol. 256, No. 24, 1986), changing the name of the table
to "Non-Hodgkin's Lymphoma in Relation to Herbicide Use .
. ." rather than 2,4-D use. However, the already extensively
publicized study was by then widely perceived as a 2,4-D study,
and it still is. How could such a significant error have occurred?
Was it simply an honest mistake? In the numerous times that NCI
has cited the Kansas study since, this writer has yet to see one
instance where NCI also cites the important correction. It seems
as if the National Cancer Institute wants the public to believe
that the Kansas study, as published, was a "2,4-D study."
The widespread media attention given the Kansas study placed considerable pressure on EPA. Since the National Cancer Institute had two other "farmworker" studies in progress (Nebraska and the Iowa-Minnesota studies) EPA decided to wait until the other two studies published, then convene a Science Advisory Board/Science Advisory Panel Special Joint Committee on 2,4-D, which would review both the toxicology and epidemiology of 2,4-D and make a "weight of the evidence" recommendation.
The NCI Nebraska farmworker study was published
in 1990. This study (Epidemiology,1(5):349-56), unlike the Kansas
study, did develop data specific to 2,4-D. It showed a non-significant
(not outside the realm of chance) association between 2,4-D and
NHL, which, according to the NCI investigators, "strongly
supported the Kansas study."
All three of the NCI farmworker studies relied heavily on information provided by "proxy" respondents. These studies are based on information obtained through questionnaires sent to populations of farmers who have been diagnosed with NHL and populations of farmers who do not have that disease. Both groups were asked about their pesticide practices over a period of 40 years or more. Many of the farmers with the disease, however, had either died or were unavailable for interview. So NCI used either those farmer's next-of-kin or their next-door neighbors to complete the questionnaire on that farmer's pesticide practices. The use of "proxy" respondents in pesticide studies is controversial. Two later studies (Boyle, 1992, and Johnson, 1993) found information on pesticide use provided by proxies to be so inaccurate that they questioned its use in epidemiologic studies involving pesticides. The Johnson study shows that proxy respondents typically overstate the use of widely known pesticides, such as 2,4-D, and understate the use of lesser-known products. NCI epidemiologists, however, took the position that the use of proxy respondents in pesticide studies actually understates the risk. In other words, according to the NCI researchers, the non-significant risk finding in the Nebraska study is actually more serious than it appears.
The authors of the Nebraska study did analyze the information provided by the self-respondents (the farmers themselves) separately from the information provided by the proxy respondents. This, in itself, should have helped answer questions about the appropriateness of proxy respondent information in this study. The information provided by the farmers themselves showed no association between 2,4-D use and cancer, significant or otherwise. Only when the information provided by the proxies is added to it, did the authors come up with the non-significant association. This is directly contrary to the NCI position that the use of proxies actually understates risk, but something the NCI researchers apparently chose to ignore. Was this simply another oversight, or was it intentional?
The Iowa-Minnesota study, like the Kansas study, developed information only on general herbicide use, not information specific to 2,4-D. Although the study was nearing completion, the authors decided to go back and reinterview a portion of the respondents about their use of 2,4-D, in order to strengthen the study. This delayed the publication of the study for several years, which delayed the convening of the EPA special review panel. However, when the study was published (1992), the reinterview information concerning 2,4-D use was, mysteriously, not included. The study, which contained more cases and controls than the Nebraska and Kansas studies combined (making it the most powerful of the three NCI studies), showed only a non-significant association between herbicide use and NHL.
A request by members of the 2,4-D Task Force to review the NCI 2,4-D reinterview data was denied, so the Task Force requested the information through the Freedom of Information Act. This data showed no association, non-significant or otherwise, between the use of 2,4-D and cancer. Additionally, it again showed that the use of proxy respondents in the reinterviews overstated risk, rather than understating risk, as NCI contends.
EPA then convened its SAB/SAP Special Joint Committee, which met in a public meeting in April 1993. The authors of the NCI farmworker studies and the canine lymphoma study presented their findings to the joint committee. They presented a summary of findings in the Iowa-Minnesota study, but without mentioning the very important 2,4-D reinterview data. Although the Task Force had received the reinterview data only days before from NCI's Freedom of Information office, it did manage to have an epidemiologist analyze the data, and, to the embarrassment of the NCI researchers present, presented the results to the SAB/SAP panel. EPA, which had waited several years for NCI to complete the 2,4-D reinterviews, was more than curious to learn the results. Again, what motivated NCI to withhold that data from the EPA joint committee? Was it simply another oversight or an example of "selective science" or biased advocacy? If so, this sort of activism is being funded by your tax dollars.
 Had a pesticide company deliberately withheld pertinent
research information from EPA, as was apparently done by NCI,
those involved would be in violation of federal law and could
face criminal charges.
Had a pesticide company deliberately withheld pertinent
research information from EPA, as was apparently done by NCI,
those involved would be in violation of federal law and could
face criminal charges.
The SAB/SAP joint committee, although concerned about the canine lymphoma study, concluded that the NCI epidemiologic studies provided insufficient evidence of a cause-and-effect relationship between 2,4-D and cancer. The EPA Carcinogenicity Peer Review Committee later concluded (January 1997), having the benefit of additional toxicologic and epidemiologic studies, that 2,4-D should remain a Class D compound. Class A com-pounds are "known carcinogens," Class B compounds are "probable carcinogens" and Class C compounds are "possible carcinogens." A class D rating means that there is insufficient evidence of carcinogenicity to place the compound in any of the three higher categories.
So while 2,4-D has survived yet one more
challenge, troubling questions remain about NCI epidemiologic
research on 2,4-D. Some have suggested that the evidence of bias
is so persuasive that the raw data from all NCI epidemiologic
studies on pesticides should be obtained under the Freedom of
Information Act for independent analysis, as was done with the
canine lymphoma study and the Iowa-Minnesota farmworker study.
Certainly the results of those efforts are very revealing. In
the meantime, NCI epidemiologists have initiated the 20-year,
multi-million-dollar Agricultural Health Study (AHS), which looks
at the effect of pesticide use on farmers, spouses, and their
children. Can we expect a completely unbiased study? Past history
would suggest not. Estimates of total cost of the AHS vary, but
some say the eventual cost will exceed $100 million of taxpayer
money. Would not this money be better spent on basic cancer research?
After all, the American Cancer Society says that less than two
percent of all cancers are caused by pesticides. No one knows
just how much less than two percent. Yet 30 percent of all cancers
are caused by smoking and 35 percent caused by lifestyle (mainly
diet). NCI epidemiologists have now spent millions of taxpayer
dollars in their search for the elusive cause-and-effect relationship
between 2,4-D use and cancer. Perhaps it's time to initiate an
FOIA request into how much of a role the NCI "2,4-D studies"
played in NCI's justification for the taxpayer funding of the
multi-million dollar Agricultural Health Study. As the saying
goes, it usually pays to follow the money trail.
In the meantime, some researchers and organizations are becoming alarmed at the apparent anti-2,4-D bias among NCI epidemiologists. A recent editorial in the Wall Street Journal (August 10, 1999) refers to NCI's "jihad against the widely-used lawn herbicide 2,4-D" and the Cato Institute, a Washington, D.C. think tank, is in the process of publishing an extensive paper on the NCI/2,4-D saga.
Copies of the Cato Institute paper or any of the studies mentioned here can be obtained by calling the Task Force at 1-800-345-5109, and additional information on 2,4-D is available on the Internet at www.24d.org.
This article originally appeared in the
November 1999 issue of Wheat Life (Vol. 42, No. 10), and appears
here by permission of the magazine and the author. Donald Page
can be reached at (800) 345-5109 or donpage@24d.org.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Concerns about environmental contamination and pesticide residues on food have led to increased regulation of pesticide use and encouragement of Integrated Pest Management (IPM). IPM strategies tend toward reducing the use of conventional pesticides in favor of reduced-risk pesticides and non-chemical approaches to pest control. Petroleum spray oils (PSOs) show increasing promise as part of an IPM system; they have demonstrated reduced-risk properties and are also cost-effective and safe and easy to apply.
PSOs have been used for pest control for well over one hundred years. Applied for cool-season control of pests that overwinter on trees and shrubs, they have historically been referred to as "dormant oils." Those used during the growing season have been referred to as "summer oils."
PSOs kill insects and mites through suffocation by smothering. They are effective in all stages of insect growth, including the egg stage. The oils are often tank-mixed with an organophosphate or pyrethroid insecticide, where they aid in coverage and increase the penetration of insecticide into waxy plant surfaces.
Several key chemical properties characterize PSOs and affect their pesticidal activity (Pearce et al. 1942, Jacques and Kuhlmann in press), including chemical composition, carbon number, distillation temperature, degree of refinement, and viscosity.
(a) Chemical Composition. PSOs have four basic chemical structures: paraffin, naphthene, olefin, and aromatic (illustrated in Figure 1). Olefin and aromatic structures contain double-bonded atoms (as indicated by the dual lines connecting some of the carbon (C) atoms in the illustrations of their structures), rendering them less stable and more phytotoxic. Naphthene has no double bonds, but is more easily broken and therefore reactive (thereby potentially phytotoxic) than paraffin. Paraffin is the most desirable structure; it is the least phytotoxic and has good viscosity for application (see "viscosity," p. 6). Virtually all oils are a combination of two or more of the chemical structures, so, for practical use as a PSO, an oil should be at least 60% paraffin (Simanton and Trammel 1967). For summer (in-season) use, the paraffin content should be even higher. Improvements in refinement techniques have enabled us to produce PSOs with higher paraffin levels.
|
|
|
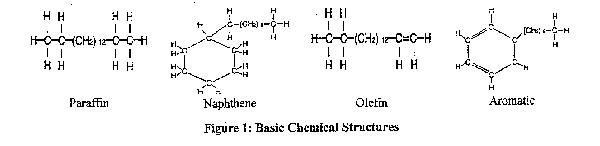 |
(b) Carbon Number. PSOs all have pesticidal properties but also have phytotoxicity potential. Pesticidal activity generally increases with increasing paraffin content, up to a carbon number of approximately 23. Plant phytotoxicity becomes a chronic problem when the carbon number is larger than 25 (Jacques and Kuhlmann in press). Staying within this narrow range of effectiveness, most commercially available oils have carbon numbers in the 20-to-25 range.
(c) Distillation Temperature. U.S. regulatory agencies qualify PSOs with the ASTM D 1160 vacuum-distillation method. This technique measures the temperature at which 10, 50, and 90 volume percent of the PSO distills off under a vacuum of 10 mm of Hg. If the 50% distillation temperature is 224 ± 4.5° C (435 ± 8° F) and the difference between the 10% and 90% distillation point, called the "range," is less then 44.4° C (80° F), then the PSO is called a "435 narrow-range oil" (Chambers 1992). Oils with this property are less reactive, therefore less phytotoxic, and more appropriate for in-season use.
(d) Degree of Refinement. Degree of refinement is gauged by a spray oil's unsulfonated residue (USR). USR is the volume of the oil that does not react with N sulfuric acid. When an oil is exposed to N sulfuric acid, the unsaturation groups or carbon double bonds in molecules react or become sulfonated. Aromatic compounds are also consumed in the sulfonation reaction. The final oil volume, expressed as a percent of the original amount of oil, is the USR. A minimum USR of 92 is recommended for protection against phytotoxicity (Simanton & Trammel 1996, Reihl 1969). It should be noted that pesticide activity does not increase above 95 USR (Chapman 1967).
(e) Viscosity. The flow characteristics of an oil and concentration of deposits on a plant during treatment is important to the safety of the plant. Lighter oils flow more freely and spread more evenly over the plant surface, making them more appropriate for warmer temperatures and times of greater foliage, while heavier oils, which tend to bead up more on the plant, should be used primarily during dormancy.

While growers have commonly used PSOs to
manage overwintering pest populations during the dormant season,
they have been reluctant to use them during the growing season
because of their phytotoxicity. Improvements in oil refinement
technology have resulted in products safer for in-season (warm
weather) application.
Most of today's pest-control PSOs are highly refined. Many can
be used throughout the entire season if the label instructions
are followed carefully. During warm weather, plants should be
irrigated prior to PSO application. Sprays should be applied when
temperatures are cooler, e.g., early mornings or cloudy days.
Allow plant tissues to dry after rain or irrigation before applying
PSO, and avoid application if humidity is expected to remain over
90% for 36 hours or longer. Avoid applications during shoot elongation.
Do not apply oils when temperatures are above 100°F or below
40°F. Horticultural oil will work only if the pest is present
on the plants you are spraying. Prophylactic applications are
of no use and are not recommended.
Table 1 lists examples of "unclassified petroleum oils" and "mineral oils" registered for agricultural use. These heavier, less refined oils are intended for use primarily during dormant season. Unclassified petroleum oils are also registered in combination with conventional pesticides and commonly used as tank mixes (Table 2).
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
|||||
|
|
|||||
|
|||||
In addition to horticultural mineral oils, some registered pesticidal oils are derived from oilseeds such as castor, cotton, and soybean. While such vegetable oils can be quite effective in controlling scales and other insects, they are rarely used except by organic farmers because of their potential to cause phytotoxicity. Primarily, these materials are used as refined spray adjuvants (Table 3).
|
||||||
|
|
||||||
|
||||||
Paraffin-based oils and refined petroleum distillates account for less than 10 percent of the 'mineral oils' and 'unclassified petroleum oils' currently used (California Department of Pesticide Regulation 1996). The 'refined petroleum distillates' category includes several products that are used as insecticides or fungicides. These products, examples of which are shown in Table 4, are more refined and safer to use during the growing season and in warmer temperatures.
|
||||||||||||
|
|
||||||||||||
|
||||||||||||
With the implementation of the Food Quality Protection Act of 1996 and continuing concern for environmental quality, worker safety, and pest resistance to pesticides, IPM continues to be promoted as an important approach for controlling pests in both agricultural and urban settings. Horticultural mineral oils present a reduced-risk approach to controlling pests in keeping with the movement toward more biologically based systems. We know from currant usage that oils can act as direct controls for scale insects, mites, and aphids in some crops. Research is needed to improve efficacy and further reduce phytotoxicity. The present uses can no doubt be expanded to include other crops, more acreage, and other insects such as leafhoppers, mealybugs, and leafrollers. PSOs' fungistatic properties also make their use in disease control attractive; research is needed in this area. Pear scab and powdery mildew present two examples of diseases for which horticultural mineral oils could provide a supplemental control approach.
Horticultural mineral oils have long been used as adjuvants with conventional pesticides to improve the level of efficacy of these products. Their use in combination with reduced-risk pesticide products for insect and disease control should be more widely tested to see if these materials can be used more effectively, or at lower rates and therefore more economically. Finally, research on horticultural spray oils should be conducted as part of the development of reduced-risk systems similar to those using pheromone mating disruption.
PSOs are already applied to a wide variety of crops. Specific guidelines for their use against insects, mites, and diseases have already been established on many crops, attesting to their potential and acceptance. Horticultural mineral oils are relatively inexpensive and safe to use. These products could play an important role in the establishment of future IPM systems that rely less on conventional pesticides.
Dr. Douglas B. Walsh is the IPM Coordinator for Washington State. His office is at the Irrigated Agriculture Research and Extension Center, and can be reached at (509) 786-9222 or dwalsh@tricity.wsu.edu. Dr. Frank Zalom is Director of the University of California's Integrated Pest Management Program.
Ask most farmers and they will tell you it is hard to make a living at farming. The marketplace is full of low-cost foods, many of them imported from outside the United States. Our farmers are expected to compete with the low costs of production of countries where the cost of living is much lower than our own and where there are fewer labor, water quality, environmental, and pesticide regulations and restrictions. The costs of production in the United States are increasing as our farmers meet environmental and social responsibilities; and food distributors are responding by purchasing low-cost food from countries that do not have these same responsibilities. Washington agriculture, just as in the rest of the nation, is in transition as the family farm struggles to maintain its viability in this market place.
Some farmers are remaining viable by producing
alternative crops. Alternative crops are simply crops that are
not generally grown in an area, or they are crops that are new
to your marketplace. For example, wheat in western Washington
could be considered an alternative crop. Vegetable soybeans are
also considered an alternative crop in the United States though
they have been grown in Asia for centuries. At a quick glance,
some alternative crop ideas may seem ridiculous or unmarketable.
But ask yourself, were canola oil or salad mixes in your diet
fifteen years ago? Probably not. Yet today these are common foods
in cupboards and on tables throughout the country.
Testing the market potential of a new crop involves taking calculated risks. Calculations should involve a thorough and realistic assessment of the production needs of the proposed crop. Will specialized (hand) labor or new equipment be needed? What will planting, cultivation, harvesting, and processing involve? How will the product be distributed, and to whom will it be marketed? How will it be marketed? Taking the time to plan and consider these issues will differentiate between calculated risk and foolhardiness.
To minimize start-up costs and potential losses, start small. Test the production environment and the marketing strategies on a manageable scale before making large investments or planting a large area to an alternative crop.
Producing an alternative crop can be challenging; often, little information is available on the crop or on how it will grow in your area. In the Washington State University Cooperative Extension Agricultural Systems program, we have been working with farmers, colleagues, and seed companies to test several alternative crops, including edamame (also called vegetable soybeans and sweet beans), baby corn, pea shoots, and bamboo shoots. In cooperation with farmers, we have conducted variety trials to determine whether each of these crops is suited to the region. Seed companies and university plant breeders have generously provided seed for our trials.
 Some crops, such as
baby corn and pea shoots, have well developed agronomic practices;
they are versions or parts of well established crops with which
we are all familiar. Baby corn can be harvested from a regular
sweet corn planting, approximately three days after the silks
first appear; pea shoots are the top four to six inches of pea
vines. In these cases, most or our work has focused on determining
which varieties of corn and pea are best suited to this specialized
production. We have also begun to explore value-added baby corn
products to extend the shelf life and marketing season of this
crop. In cooperation with Val Hillers and Dick Dougherty of WSU's
Food Science and Human Nutrition Department, we have tested home
canning of baby corn, the first step to developing a value-added
product.
Some crops, such as
baby corn and pea shoots, have well developed agronomic practices;
they are versions or parts of well established crops with which
we are all familiar. Baby corn can be harvested from a regular
sweet corn planting, approximately three days after the silks
first appear; pea shoots are the top four to six inches of pea
vines. In these cases, most or our work has focused on determining
which varieties of corn and pea are best suited to this specialized
production. We have also begun to explore value-added baby corn
products to extend the shelf life and marketing season of this
crop. In cooperation with Val Hillers and Dick Dougherty of WSU's
Food Science and Human Nutrition Department, we have tested home
canning of baby corn, the first step to developing a value-added
product.
Edamame and bamboo have both required much more work to determine
not only suitable varieties but also general agronomic information.
In cooperation with Del Hemphill, Vegetable
Research Specialist at Oregon State University North Willamette
Research and Extension Center, and Tim Miller, Weed Specialist
at Washington State University Mt. Vernon Research and Extension
Center, we have conducted field trials to test varieties, spacing,
fertilizer rates, and weed control methods of edamame. Edamame
are relatively easy to grow, but one of our main challenges has
been to obtain affordable seeds that are suited to our environment.
We are working with edamame breeders as well as seed companies
to broaden the number of varieties offered in the United States.
Tom Lumpkin's research program at WSU's Department of Crops and
Soils is also working on development of new edamame varieties.
As farmers begin growing this new crop, it is important that researchers
keep up with emerging pest-control issues. We anticipate that
edamame may be susceptible to some insect pests and we hope to
expand our cooperative research program to test insect control
strategies before these pests begin to limit production.
Bamboo has posed a different set of challenges to our program. First, it has been difficult to obtain crop research funding for bamboo because it does not fit with most people's vision of an agricultural crop. For many funders and farmers, bamboo has an aura of the tropics and does not seem suitable for our temperature climate (even though bamboo has been growing in the Pacific Northwest for more than a hundred years). Second, bamboo is a perennial crop that may require five to eight years to establish in our region before it can be harvested. Thus it takes years of field work and maintenance before we will see any yield results. And finally, there has been some concern that bamboo will "escape" and "invade" the region, particularly in wetland areas. Yet bamboo was introduced into the region in the late 1800s and shows no sign of becoming invasive or a weed. We feel that bamboo has very high potential as a sustainable food and fiber crop for our region. It is interesting to note that bamboo research conducted by USDA scientists in the Southeast in the first half of this century indicated that bamboo could sustain multiple harvests, and the production potential was greater than for many pine species. In cooperation with the American Bamboo Society, local farmers, and WSU researchers Andy Bary and Craig Cogger, we have established a bamboo variety trial that will enable us to begin assessing the food and fiber production potentials of bamboo. Also of much interest to us is the potential to utilize bamboo for recycling animal waste, as bamboo is thought to be a high nitrogen user.
Marketing an alternative crop can be especially difficult because consumers may not know how to use an unfamiliar food. When launching a new alternative crop, it is helpful to provide customers with take-home information such as recipe cards. One side of the card can be used for cooking and eating instructions, while the other side can be used for general information about the crop-people are curious and want to know. Recipe-sized cards are small, therefore handy for the consumer and thrifty for the marketer. It is important to provide first-time buyers with correct cooking directions to avoid unpleasant eating experiences and encourage repeat purchasing. For example, if consumers eat the pods of edamame, it is unlikely they will try this food again. Edamame do not have edible pods, and although eating the pods will not cause any health problems, they are not highly palatable. When possible, provide free taste samples to entice customers.
In cooperation with Gayle Alleman, Registered Dietitian at WSU Cooperative Extension Kitsap County, we have developed marketing brochures and recipe cards that present nutrient and phytochemical content of the alternative crops we are fostering. These materials are available for growers to distribute to customers. In cooperation with Mark Musick, Farm Advisor at the Seattle Pike Place Market, we have promoted these alternative crops at workshops and taste testings targeted at local chefs and food writers. By increasing the demand for these crops, we hope to increase the opportunities for production.
An ideal approach to investigating the production and marketing potentials of alternative crops includes an interdisciplinary research and extension program integrated with farmers and marketers. In Washington, we are striving to build these relationships. It is our hope to continue our studies of the alternative crops we are currently investigating and to expand our program to include other crops that show promise for our region.
For information on producing edamame, baby corn, pea shoots, and bamboo, visit our website at http://agsyst.wsu.edu. The website also includes examples of our marketing brochures, information on pest management and other production issues, and links to many helpful resources.
Dr. Carol Miles is an Agricultural Systems
Agent with WSU Cooperative Extension. She can be reached at (360)
740-1295 or milesc@wsu.edu.
During 1995 and 1996, an innovative Oregon wholesaler, Fall Creek Farm & Nursery, began propagating lingonberry plants and selling them as an ornamental product. Fall Creek, known for its blueberry nurserystock, is located in Lowell, about twenty miles from Eugene.
 The lingonberry
is a small, red fruit grown on bushes less than a foot tall. It
is also known as cowberry, partridge berry, mountain cranberry,
rock cranberry, dry-ground cranberry, lingen, lingberry, fox berry,
and red berry. The name "lingonberry" originated in
Sweden and is used in Canada and America. In Newfoundland it is
called partridge berry. The Scandinavian market prefers the name
cowberry.
The lingonberry
is a small, red fruit grown on bushes less than a foot tall. It
is also known as cowberry, partridge berry, mountain cranberry,
rock cranberry, dry-ground cranberry, lingen, lingberry, fox berry,
and red berry. The name "lingonberry" originated in
Sweden and is used in Canada and America. In Newfoundland it is
called partridge berry. The Scandinavian market prefers the name
cowberry.
I separate the lingonberry into two classifications,
the wild (Vaccinium vitis-idaea Minus) and the European
domestic (Vaccinium vitis-idaea). The wild lingonberry,
"Minus," is found across the Northern Hemisphere in
Alaska, Saskatchewan, Nova Scotia, Newfoundland, Labrador, and
the Scandinavian countries. The plant is quite short, 3 to 6 inches
tall, and produces fruit on a single bloom.
Domestic or European lingonberries are plants that have been cross-bred; some originally came from Europe. This plant can grow to 8 to 12 inches tall, has two blooms and produces more fruit than the wild variety.
The Scandinavian countries pick and use over 100 million pounds of "cowberries" per year, which they use in jams, jellies, preserves, concentrates, and liquors; the berries are also sold fresh.
Cultivated lingonberries in the United States can be traced largely to the efforts of Dr. Elden Stang. Dr. Stang went to Finland in 1987 and worked for four months with several Finnish scientists, bringing back many Scandinavian cultivars. He also brought back 7000 seeds. Here, he continued his work on breeding, which resulted in two new varieties, "Regal" and "Splendor."
Initially, the U.S. lingonberry industry began in Wisconsin, but dwindled due to lack of marketing efforts, difficulty in harvesting, and the double-blooming tendency. Currently, there are two growers and one nursery in Wisconsin; these are relatively "new" growers.
In the mid-1990s, the lingonberry began to make commercial inroads again in nurseries in Oregon, Wisconsin, and Michican, primarily as an "edible ornamental" plant; ornamental sales have been strong since 1996. Here in Oregon, it started when growers in and around the Willamette Valley, Oregon, including Dave Brazelton of Fall Creek Farm & Nursery, began asking, "Will lingonberries grow commercially in the Willamette Valley?" It was a good question. When they asked Extension people at Oregon State University, we didn't have the answer.
 I contacted
state berry specialists around the United States. No one knew
much about lingonberries.
I contacted
state berry specialists around the United States. No one knew
much about lingonberries.
Next, I dispatched a research technician, Karen Ailor, to conduct an extensive literature search. Six months of research yielded fifty-six citations. Of those, thirty-eight were production and marketing articles. A web search resulted in 735 hits, but most were jam, jelly, and food recipes. We compiled these efforts (minus the recipes) to produce Growing Lingonberries: A Survey of the Literature. Chapters included background, cultural conditions, bloom, harvest, diseases, other pests, and references. Our second product, Lingonberry Harvest and Marketing: A Survey of the Literature, reviews the harvest and post-harvest aspects. As very little correlates in the sparse body of research available, both publications make heavy use of direct quotations from the literature. These surveys are available to the public for $3.00 each. They can be purchased from the OSU/Lane County Extension Office, 950 West 13 Avenue, Eugene, OR 97402 or by calling the Natural Resource secretary at (541) 682-7308 or (800) 872-8980.
Three test plots were planted in the Willamette Valley in the spring of 1998. We surveyed the plots for insects, diseases, and weeds. During the year the only insects seen were cucumber beetles that moved in after the grass fields were harvested. They tried to feed on the waxy leaves, but no damage was reported. We suspect that lingonberries will become host to many of the same insects that affect other small fruits such as cranberries, blueberries, and raspberries.
Several of the plants died the first year, due to the influence of both over-watering (which causes root rot) and under-watering (the plants are shallow-rooted).
We held our first lingonberry conference in March 1999. Twenty-one growers and prospective growers attended. We presented the literature search information and discussed "where people were" in the growing arena. Everyone wanted to know what everyone else was doing, so I planned a fall reconnaissance trip to Wisconsin, Michigan, Nova Scotia, and Newfoundland.
 In September
1999, I traveled to the eastern United States and Canada to look
at the production of lingonberries in the North America. I visited
Wisconsin, where U.S. production began in the 1980s, and had the
opportunity to visit with Dr. Stang. He has retired and now is
consulting with the cranberry industry. I also visited two Wisconsin
growers, whose plots, like ours in Oregon, were also three years
old or less. The biggest issue for these growers was the control
of weeds. One grower had an insect that was chewing on some of
the lingonberry leaves but it wasn't an economic issue.
In September
1999, I traveled to the eastern United States and Canada to look
at the production of lingonberries in the North America. I visited
Wisconsin, where U.S. production began in the 1980s, and had the
opportunity to visit with Dr. Stang. He has retired and now is
consulting with the cranberry industry. I also visited two Wisconsin
growers, whose plots, like ours in Oregon, were also three years
old or less. The biggest issue for these growers was the control
of weeds. One grower had an insect that was chewing on some of
the lingonberry leaves but it wasn't an economic issue.
Since lingonberries are a "micro" market, pesticide registration may not happen. Therefore, test plots are being run without the use of pesticides to see if they can produce lingonberries with minimal or no pesticides. Weed control will be the biggest issue. Currently, growers apply six inches of mulch over the plant, within the row, to smother small weeds. This mulch reduces the growth of weeds but does not eliminate them. In between the rows, a slow-growing grass is planted, then mowed three times per year.
From Wisconsin, I went to Michigan, where I visited three nurseries that propagate lingonberries. The plants are propagated by stem cuttings and micro-propagation techniques. All growers see great potential for lingonberries in the next few years.
Next, I visited two growers in Maine. The first one was an energetic young man who is really excited about growing lingonberries. His plots were also young but in good condition. The second grower had plots of both the wild and the European varieties, each three years old or less. The elusive lingonberry patch with lots of berries still had not been found.
Then I was off to the agricultural research
station in St. John's, Nova Scotia, to talk with Dr. Andrew Jamieson.
He had an older plot of lingonberries and had been harvesting
it for over four years. He had some nice yield data from eight
different varieties. The only other grower in the area had a three-year-old
plot with great-looking plants and good weed control.
The visit to Newfoundland was my last chance to see a big field of ripe, red lingonberries. Boyd Penney and Dr. Samir Debnath took me on a whirlwind trip around Newfoundland looking at the wild cultivars. They had collected the wild lingonberry and planned to do some variety breeding and micro propagation. Mr. Penney's plots were over four years old but the berry production was still light.
Dr. Peggy Dixon, an entomologist in the Newfoundland area, is conducting research on the lingonberry fruitworm, a moth-larva pest that infests the fruit and makes it unmarketable. She has used pheromone traps to monitor the moth.
I learned a great deal from my wide-ranging tour of North American lingonberry producers, but, as with any crop in the early stages of commercial production, perhaps more questions were raised than were answered. In particular, four issues still loom large:
We held another lingonberry conference October 1999 in Aurora, Oregon. This time, forty-two growers and prospective growers attended the conference. They learned that Oregon and Washington growers are just as far along as others in North America in producing lingonberries.
Ross Penhallegon is a Horticulture Extension Agent for Oregon State University. He can be reached via e-mail at ross.penhallegon@orst.edu.
|
A funny thing happened to me on my way through graduate school. For my graduate assistantship I was a PCO (pest control operator), spraying and baiting for vermin in the dormitories, campus buildings, and teaching hospital. Yes, that's right, I said hospital. Burned in my memory like insecticide deposits on cockroach tarsi is a truly remarkable experience given the sensibilities of today's world. I got a call one evening to spray for cockroaches in the pediatric intensive care ward. Our weapon of choice at that time was the still comparatively new Dursban containing the active ingredient chlorpyrifos, which was first registered in 1965.
Now before you treat me as some kind of
pariah, you need to know the time was 1973 and the place was the
University of Florida. The cockroaches in Florida were nearly
big enough to carry off the rats we also had trouble with. OK,
not really, but the American cockroach (Periplaneta americana)
is a formidably big domestic insect with some limited flight capability,
and doctors don't like seeing them crawling around sick infants.
Cockroaches are nasty looking little beasts whose populations can quickly explode to overwhelming numbers. Cockroach droppings and body parts have been associated with asthma syndromes in young kids and adults (6, 7, 11). So, in the absence of a children's protection law like the Food Quality Protection Act (FQPA), the doctors in Florida may have felt that the neurotoxic properties of insecticides were a remote hazard compared to cockroaches crawling around a supposedly clean hospital ward.
How the times have changed. After almost thirty-five years on the market, chlorpyrifos is being saddled by EPA with an extra safety factor to protect infants and children from excessive exposures. But the benefits of using chlorpyrifos for cockroach control was not one that had to be balanced by the EPA when they ruled that scientific studies suggested that infants and children may be more susceptible than adults to the neurotoxic effects of chlorpyrifos.
Ostensibly, the EPA was standing on sound legal ground when it imposed an extra threefold safety factor. Besides, things could have been worse-the agency could have taken the option for a tenfold factor. The extra factor was over and above the routinely imposed 100-fold factor that is used to derive an acceptable level of exposure or Reference Dose (RfD) for a pesticide.
Specific provisions of the FQPA were designed to minimize the hazards of pesticide residues to infants and children. For example, the FQPA directs EPA to consider "available information concerning the special susceptibility of infants and children to the pesticide chemical residues, including neurological differences between infants and children and adults, and the effects of in-utero exposure to pesticide chemicals." To support their pesticide's registration, therefore, manufacturers must submit results of neurotoxicity, developmental toxicity, and reproductive toxicity tests (3). EPA assesses whether infant rats might be more sensitive than adults by comparing the dose causing no observable effects (the NOEL) for specific physiological effects like weight loss, tissue damage, or enzyme levels.
 In addition to the
raw hazards of a pesticide, the FQPA directs EPA to consider children's
degree of exposure. EPA must assess the risk of a pesticide based
on con-sumption patterns peculiar to children that might result
in disproportionate dietary exposure compared to adults.
In addition to the
raw hazards of a pesticide, the FQPA directs EPA to consider children's
degree of exposure. EPA must assess the risk of a pesticide based
on con-sumption patterns peculiar to children that might result
in disproportionate dietary exposure compared to adults.
Finally, EPA must "ensure that there is a reasonable certainty that no harm will result to infants and children from aggregate exposure," i.e., residues around the home as well as in food and water. Because of its heavy use for urban insect control, chlorpyrifos has the dubious distinction of being the first FQPA-era candidate to have a sufficient database on aggregate exposure.
Once the EPA has formed an opinion about the risks kids might face owing to disproportionate exposures and/or special sensitivity, the agency is mandated to "publish a specific determination regarding the safety of the pesticide chemical residue for infants and children." And this is where things got hairy for chlorpyrifos.
Nodding to a popular assumption that kids were under greater threats from pesticide exposure than adults, the FQPA in an unprecedented mandate literally specified a numerical margin of safety to use when ensuring the reasonable certainty of no harm from aggregate exposure. Thus, EPA must automatically apply "an additional tenfold margin of safety for the pesticide chemical residue and other sources of exposureto take into account potential pre- and postnatal toxicity and completeness of the data with respect to exposure and toxicity to infants and children."
This FQPA provision, commonly called the FQPA 10X factor, rallied certain advocacy groups to call for EPA's head when the Agency seemed unwilling to apply it to many OP insecticides. But this reaction belied the rest of the FQPA language that presumably some of those groups' lobbying efforts might have influenced in the first place. The 10X factor paragraph also states "Notwithstanding such requirement for an additional margin of safety, the [EPA] Administrator may use a different margin of safety for the pesticide chemical residue only if, on the basis of reliable data, such margin will be safe for infants and children." So EPA was justified in recommending a lower 3X safety factor.
Application of the 10X factor to a pesticide
residue regardless of exposure pathway automatically reduces by
tenfold all permissible exposures, shrinking the metaphorical
risk cup. Add this to a NOEL that has been divided by the first
safety factor of 100, and that cup becomes tiny. Ironically, the
first 100-fold factor applied to the NOEL already accounts for
differences in susceptibility among different age groups.
Interpreting literally the "reliable data" loophole of the 10X factor mandate, Dow AgroSciences (DAS) is naturally going to fight tooth and nail for consideration of its data showing fetal and neonatal (i.e., infant) rats are not more susceptible to chlorpyrifos than their parents. EPA, while admitting that the DAS data looked sound, chose to halfway ignore it by jumping into the published scientific journal literature and pulling out the plum of increased neonatal rat sensitivity at acutely lethal doses. But DAS, being a company with a long tradition of encouraging its scientists to publish their results, had its own peer-reviewed journal articles supporting its case.
So what prevailing hypotheses make EPA so jumpy about possible increased sensitivity of children, and DAS so sure the data indicate no need to worry?
In the chlorpyrifos risk assessment, EPA cited several studies in the scientific literature that indicated suckling rats are indeed at least several times more susceptible to the acute effects of chlorpyrifos. The key word here is acute; the studies cited were testing either lethal doses or maximum tolerated doses (MTDs). While outright death was not observed at MTDs, and weight did not decrease by more than 10%, there is no doubt that brain AChE was significantly inhibited. In some studies (9, 10), nonlethal but telltale signs of poisoning had occurred-tearing, salivation, and tremors-indicating that MTDs are still quite toxic. Many studies administered doses by sub-cutaneous (under the skin) or intraperitoneal (into the abdomen) injection. Such unconventional exposure routes expose an organism to a very large dose all at once, bypassing the protective layer of the skin or the much slower absorption into the bloodstream from the intestine.
 Such drastic doses
and methods of exposure are part of what I like to call mechanistic
toxicology studies. Their objectives are to characterize physiological
responses and their biochemical bases. Occasionally, doses are
used that elicit no response in an attempt to track subtle changes
in biochemistry. The objective of regulatory toxicological studies,
on the other hand, is to find the NOEL for the most sensitive
biochemical or physiological response.
Such drastic doses
and methods of exposure are part of what I like to call mechanistic
toxicology studies. Their objectives are to characterize physiological
responses and their biochemical bases. Occasionally, doses are
used that elicit no response in an attempt to track subtle changes
in biochemistry. The objective of regulatory toxicological studies,
on the other hand, is to find the NOEL for the most sensitive
biochemical or physiological response.
The DAS studies submitted for EPA review used doses spanning from the NOEL through those known to produce an effect. Chlorpyrifos doses were given to a mother rat during pregnancy and for 11 days after birth to expose both the fetuses and the suckling newborns (neonates). The parental NOEL was always lower than the NOEL for the offspring (Table 1). In other words, the neonates were less susceptible than the adults for the most sensitive endpoint examined. Endpoints ranged from enzyme inhibition to brain histopathology and functional behavior, all effects that would be predictive of adverse neurodevelopment.
|
|
||||
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12 weeks |
|
|
In nearly every case EPA used to support its conclusion that infants may be more susceptible than adults, doses were incredibly high relative to real world exposures. For example, in one cited study 1- to-4-day-old rats were injected subcutaneously with 1 mg/kg/day of chlorpyrifos (14), the MTD previously observed not to cause outward signs of anticholinesterase toxicity. Nevertheless, brain AChE was significantly inhibited. More importantly, the exposure was nearly 800 times greater than aggregate (dietary and residential) exposure modeled as well as measured at the 99.5th and 100th percentile, respectively (4).
Recent research compared neurochemical effects of chlorpyrifos and methyl parathion in neonatal and adult rats, and it sheds some light on the discrepancy in observations between the higher dose mechanistic studies and the lower dose regulatory studies (8). Each insecticide was subcutaneously injected into neonatal and adult rats for 7 or 14 days in a row with doses equivalent to about 20% of their MTDs. Brain cholinesterase and receptor binding inhibition were similar in neonatal and adult rats exposed to chlorpyrifos, but reversed to control levels more quickly in neonates. Neonates were always significantly more sensitive than adults to the effects of methyl parathion exposure.
Thus, neonatal rats respond differently to different OPs. Neonatal rats are more susceptible than adults to the lethal effects of acute high doses of chlorpyrifos, but are less sensitive to the subacute intermittent doses and equally sensitive when exposed to subacute daily doses. In essence, the level of dose determines the differential sensitivity for chlorpyrifos but not for methyl parathion.
Curiously, EPA gave a cursory nod to an intriguing new area of research regarding possible effects of chlorpyrifos on neuronal cell replication and growth. Exposure of neonatal rats to subcutaneous injections of 1 or 2 mg/kg chlorpyrifos resulted in an inhibition of DNA synthesis and abnormal functioning of one component of the cell cycle control system called adenylyl cyclase (14, 15). However, significant brain cholinesterase inhibition occurred immediately after dosing, so it is doubtful whether the effects were truly more sensitive endpoints than plasma cholinesterase (ChE) inhibition (8).
On the other hand, a hypothesis currently in vogue is that chlorpyrifos may affect neurodevelopment through inhibition of neuronal cell (neurite) branching and rate of growth (13). A recently released study used a special nerve cell culture (PC12) and showed that chlorpyrifos and its nontoxic metabolite TCP can reduce overall neurite growth without significant inhibition of ChE activity (2). The research suggests that chlorpyrifos and other anticholinesterase compounds react at a molecular site different than the one responsible for inhibiting the enzyme.
One of the problems with cell culture studies is the difficulty of relating the concentration of a toxicant in the dish to the dose given to a rat. The PC12 cell study did show a definitive NOEL for neurite response to chlorpyrifos and TCP. The authors claimed that the concentration adversely affecting neurite growth was similar to brain levels of TCP reported in a study where pregnant rats were fed chlorpyrifos for four days at a rate of 3 mg/kg/day (5). This oral dose is nearly 2500 times greater than the highest aggregate exposure to children (4).
The reported PC12 cell culture study may not have realistically
reflected the concentration of chlorpyrifos or TCP that would
be in the neonatal rat brain following exposure to a dose equivalent
to the developmental neurotoxicity NOEL (see Table
1). TCP was not detected in blood from five-day-old lactating
neonatal rats whose mothers were exposed to 1 mg/kg/day chlorpyrifos
during pregnancy and for 10 days after birth. Exposure of a neonate
rat during lactation is most relevant to human fetal development
because the newborn rat brain is equivalent to the developmental
stage of a human brain during the third trimester of pregnancy
(14).
EPA claims that they will assess risk and make registration decisions using a "weight-of-the-evidence" approach. While concluding that DAS' data indicated no increased sensitivity of infants relative to adults, the agency chose to delve into the published scientific literature and find the "weight" it needed. Yet, most of the studies it did cite actually showed that neonatal rats were only more susceptible to lethal acute exposures but less susceptible at nonlethal intermittent or daily exposures. Three recently published papers have examined the same literature among other pieces of evidence and have thrown their "weight" behind removal of an extra FQPA safety factor (1, 4, 12).
With transparency seemingly ruling the EPA lately, anyone can read the chlorpyrifos risk assessment documents (http://www.epa.gov/oppsrrd1/op/status.htm) and make up their own mind. EPA has invited submission of comments for its consideration as it prepares the final re-registration decision. I'm waiting to see if the Tale of Two Sciences will continue.
Dr. Allan S. Felsot is an Environmental Toxicologist with WSU's Food and Environmental Quality Lab. He can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
The Food and Environmental Quality Lab (FEQL) Advisory Board met November 23 at the Washington State University Tri-Cities (WSU TC) campus. The FEQL is a research and information entity established in response to 1991 Washington State legislation and located at WSU TC. The Advisory Board meets approximately twice yearly. Objectives of this meeting included election of officers, recap of 1999 accomplishments, and discussion of objectives for the coming year.
Dean James Zuiches, College of Agriculture and Home Economics, Washington State University, called the meeting to order. John Brown, Chair of the Entomology Department, accepted the nominations and unanimous election of Scott McKinnie as Chair and Marilyn Perkins as Vice-Chair of the board. Perkins, with the League of Women Voters, represents the consumer constituency.
A two-page summary of FEQL accomplishments and activities for the 1999 calendar year was distributed and program members spoke about their work over the previous year. Allan Felsot outlined his three primary research projects, applying environmental toxicology to issues of efficacy and environmental stewardship. Catherine Daniels briefed the group on her work with the Pesticide Information Center (PIC) and explained the funding crisis currently faced by PIC. PIC's funding has come almost exclusively from the Pesticide Impact Assessment Program (PIAP) in the past, and this federal funding, through a recent act of Congress, has gone from formula-funded to competitive-funded. While the FEQL's PIC will compete for the new funds, the resulting process creates a funding gap effective January 1, 2000. Chair Scott McKinnie said that he would draft a letter in support of the continuation of funding for PIAP projects. Doug Walsh detailed the changes and expansions in his job over the past year, including the IR-4, IPM, Washington State Commission on Pesticide Registration, and research projects.
Jeff Jenkins of Oregon State University recapped the history of FEQL's role as a catalyst for cooperation between Washington, Oregon, and Idaho. The PIAP state liaisons and IR-4 area coordinators for the Pacific Northwest states, along with British Columbia regulators, had met the previous day to discuss possible cooperative efforts between the states in applying for the new competitive funding that will replace PIAP. Dr. Jenkins emphasized the connection between PIAP and IR-4, giving the example of how the crop profiles mandated by PIAP clearly support IR-4 projects.
Carol Ramsay of WSU's Pesticide Education Program (PEP) began the afternoon describing her future plans for PEP. These included on-line recertification training, collaborations with Pacific Northwest Agricultural Safety and Health center, expanded consumer education, validation of certain state license exams, and launching of a comprehensive National Pesticide Educational Resources Web Site.
John Brown told the group that all the current FEQL faculty members (Catherine Daniels, Allan Felsot, Doug Walsh) are now members of the Department of Entomology. He described how his own experience in teaching courses on the Toxicology of Pesticides, the Pesticide Applicator Training of Carol Ramsay, and the ecotoxicology work of John Stark are complementary to the FEQL concept, and explained that he, Ms. Ramsay, and Dr. Stark would be functioning as part of FEQL moving forward into 2000. These six faculty members will soon be joined by two new hires, an Analytical Chemist (next July) and an Endowed Chair of Urban Pest Management (autumn of 2002). This group of eight will form the foundation for a graduate training center to be located on the WSU TC campus.
Dr. Brown introduced three potential areas of emphasis that have been discussed among FEQL members for future consideration: buffer zones, genetically modified organisms (GMOs), and pesticide exposure risk assessment. Allan Felsot led a discussion during which the Advisory Board supported the FEQL becoming involved in evaluating buffer zone strategies. The board also supported FEQL's continued involvement in pesticide exposure risk assessment. However, the Advisory Board recommended that the FEQL not pursue programs addressing Genetically Modified Organisms at this time. The Advisory Board meets again in April, 2000.
Scott McKinnie is Executive
Director of FarWest Fertilizer & AgriChemical Association
and the Chair of the FEQL Advisory Board for 2000. He can be reached
at (509) 838-6653 or scottm@farwestfert.com.
In production settings (e.g. agriculture, lawns, golf courses, and home gardens), nitrogen (N) is the "make-or-break" plant nutrient. Lack of sufficient N, especially at key growth stages, produces a weak, stunted, yellow plant. Conversely, too much N can also adversely affect plant growth and development. While soils contain N, most do not contain enough to produce both the quantity and quality of growth desired. Therefore, most growers need to add some form of nitrogen fertilizer.
Nitrogen exists in any given soil in both organic and inorganic forms. The amount of each is an ever-changing quantity, due to a dynamic process known as the nitrogen cycle.
Organic N is converted naturally to inorganic N through decomposition, a process by which microorganisms (bacteria, fungi, actinomycetes) consume organic materials, releasing ammonium and nitrate (forms of inorganic N). The microorganisms that break down organic matter can at times require more nitrogen than is readily available to them. In this event, the soil microorganisms will utilize inorganic nitrogen (nitrate, ammonium), converting it into an organic form. For any of these processes to take place, the soil must be warm enough and moist enough for the organisms to be active.
To be of use to plants, nutrients must be soluble. Soluble N is available to plants predominantly in the inorganic forms of nitrate or ammonium. Most plants take up both forms of inorganic N, but some plants will take up only nitrate or only ammonium. The plant does not care if the nitrate or ammonium it needs comes from the breakdown of organic matter or from a synthetic fertilizer. As long as the soluble N is there, the plant will take it up and use it. In fact, it will keep taking it up whether it really needs more N or not.
Likewise, the microbes acting on the nitrogen in the soil cannot differentiate whether the inorganic N originally came from fertilizer or organic matter, either. As part of the nitrogen cycle, they can (and do) convert inorganic (fertilizer) N into organic N.
The most common way to supplement nitrogen in soil is to add soluble conventional fertilizers. Synthetic N fertilizers come in a variety of forms and sources and can contain nitrate, ammonium, or both. Such fertilizers dissolve relatively rapidly in the soil solution, becoming readily available to the plant. In the typically warm weather during a growing season, ammonium nitrogen tends to be quickly converted to nitrate by the soil microorganisms.
An alternative to conventional synthetic fertilizers is application of high-nitrogen-containing organic materials (e.g., manure, fish fertilizer) to the soil. Once added, these materials will decompose and plant-available N will be released. There are tricks to this approach. First, organic fertilizer materials do not contain plant-available N when they are applied to the soil. The rate at which the decomposition process releases plant-available N is governed by soil temperature and moisture, therefore timing is far more complex than with synthetic fertilizers.
The ability to "spoon feed" conventional ("synthetic") N and more closely predict its release of plant-available N makes it a less tricky prospect in terms of timing than an organic N source. As mentioned earlier, plants will take up soluble N as long as it is available-even beyond optimum needs. This can result in excessive vegetative growth and, in the case of fruiting plants like tomatoes, lack of fruit. Because too much N is as much of a problem as too little N, nitrogen management (timing and quantity) is important. Although there is some synchrony in the rate of N release from organic sources and plant growth, the key stages for N demand by the plant may not always coincide with N release from the organic source. Management is easier with synthetic N.
There are risks to over-application of any nitrogen fertilizer. Conventional fertilizers, due to their soluble nature, pose an increased risk of groundwater contamination when an excess is applied. The risk is reduced with organic fertilizers but it is not eliminated, particularly when high rates are applied. Organic fertilizer practices can lead to increased risk of surface-water contamination by increasing the N content in runoff-water sediment, an effect less likely to happen with conventional soluble fertilizers.
In terms of N management (quantity and timing), synthetic N offers distinct advantages. Optimum timing of organic sources is very difficult and, as a result, correct and successful use is more difficult than using conventional fertilizers. In addition, using organic fertilizers does not reduce or eliminate risk to the environment. Finally, guidelines for conventional N fertilizers are established, whereas guidelines for organic N fertilizers are few and do not have the years of research behind them.
So, are plants what they eat? Maybe not. In the case of nitrogen, plants predominantly take up inorganic nitrogen-regardless of its source of origin (organic or synthetic). This inorganic N can originate from biological based or non-biological based (i.e. synthetic) materials. Both organic and synthetic N are in constant transition between organic and inorganic forms in the dynamic soil environment. Since the plant, a living ("organic") organism, needs inorganic matter to grow and develop, using conventional synthetic fertilizers is more likely to result in successful production.
Dr. Joan Davenport is
a Soil Scientist with Washington State University in Prosser.
She can be reached at jdavenp@tricity.wsu.edu or (509) 786-2226.
Return
to Table of Contents for the January 2000 issue
Washington State University offers PRE-LICENSE courses (for those who do not have a license and need one) and RECERTIFICATION courses (for those who need to renew their current licenses). Fees are $35 per day if postmarked 14 days before the program, otherwise $50 per day. This fee DOES NOT include WSDA license test fee, which ranges from $25 to $170; for information on testing and fees, contact WSDA at (360) 902-2020 or http://www.wa.gov/agr/test/pmd/licensing/index.htm. Recertification courses offer 6 credits per day.
In reviewing the November postings in the Federal Register, we found the following items that may be of interest to the readers of Agrichemical and Environmental News.
In the November 1 Federal Register, EPA announced that it is staying the revocation of tolerances for propargite on apples and plums (fresh prunes) and is reinstating the tolerances for those commodities existing on October 18, 1999 until November 18, 1999. A final rule revoking the tolerances for apples and plums (fresh prunes) was published in the Federal Register on July 21, 1999. EPA received an objection to this rule, which requested that EPA modify the October 19, 1999 effective date for the final rule as it applied to the removal of the commodities apples and plums (fresh prunes). EPA is staying the removal of the tolerances for apples and plums (fresh prunes) effective from October 19, 1999 until November 18, 1999 in order to determine whether to grant the request for modification and, if so, for what length of time. Revocations for the remaining tolerances for apricots; beans, succulent; cranberries; figs; peaches; pears; and strawberries, subject to the July 21, 1999 rule, remain effective October 19, 1999. (Page 58792)
In the November 10 Federal Register, EPA announced that the preliminary human health risk assessments and related documents for trichlorfon, and the preliminary human health and ecological risk assessments and related documents for dicrotophos are available for review and comment. Electronically, these documents may be accessed on: http://www.epa.gov/pesticides/op/trichlorfon.htm and http://www.epa.gov/pesticides/op/dicrotophos.htm. Comments on these documents should be submitted to EPA on or before January 10, 2000. (Page 61332)
In the November 10 Federal Register, EPA announced that the revised version of the document "Estimating the Drinking Water Component of a Dietary Exposure Assessment" is now available. This document is available electronically on: http://www.epa.gov/pesticides/trac/science/. (Page 61346)
In the November 10 Federal Register, EPA announced that it was soliciting comments on the pesticide draft science policy paper entitled "Guidance for Performing Aggregate Exposure and Risk Assessments.'' This document is available electronically on: http://www.epa.gov/pesticides/trac/science/#non-occupational. Comments on these documents should be submitted to EPA on or before January 10, 2000. (Page 61343)
In the November 16 Federal Register, EPA announced that it was extending the comment period on its antimicrobial procedures proposal. The proposal would establish procedures for the registration of antimicrobial pesticides and performance standards for public health antimicrobial pesticides. The comment period is being extended to January 18, 2000 at the request of several commenters. For a summary of this proposal and more information see URL: http://www.epa.gov/oppfead1/cb/csb_page/updates/antim-regist.htm. (Page 62146)
In the November 24 Federal Register, EPA
announced it was revising the existing tolerances for glyphosate
(40 CFR 180.364) to cover applications of glyphosate in its acid
form. (page 66108)
The PNN is operated by WSU's Pesticide Information Center for the Washington State Commission on Pesticide Registration. The system is designed to distribute pesticide registration and label change information to groups representing Washington's pesticide users. PNN notifications are now available on our web page. To review those sent out in November either access the PNN page via the Pesticide Information Center On-Line (PICOL) Main Page on URL http://picol.cahe.wsu.edu/ or directly via URL http://www.tricity.wsu.edu/~mantone/pl-newpnn.html. We hope that this new electronic format will be useful. Please let us know what you think by submitting comments via e-mail to Jane Thomas at jmthomas@tricity.wsu.edu.
| Chemical (type) | Federal Register | Tolerance (ppm) | Commodity (raw) |
|
||
|
|
|
Exp. Date | ||||
| buprofezin (insecticide) | 11/3/99 page 59652 | 0.5 | cucurbits | Yes | Extension | 01-31-00 |
| Comment: This time-limited tolerance has been extended due to EPA again granting a Section 18 emergency exemption for the use of buprofezin on cucurbits for control of the silverleaf whitefly in Arizona. | ||||||
|
glufosinate-ammonium (herbicide)
glufosinate-ammonium (herbicide)
glufosinate-ammonium (herbicide) |
11/4/99 page 60112
11/4/99 page 60112
11/4/99 page 60112 |
0.5 | almond hulls |
No
No
No |
N/A
N/A
N/A |
N/A
N/A
N/A |
| 0.05 | apples | |||||
| 0.05 | cattle, goat, hogs, horses, poultry, and sheep: fat & meat | |||||
| 0.05 | eggs | |||||
| 0.1 | cattle, goat, hogs, horses, poultry, and sheep: mbp | |||||
| 0.05 | grapes | |||||
| 0.02 | milk | |||||
| 0.8 | potatoes | |||||
| 1.6 | potato chips | |||||
| 2 | potato granules & flakes | |||||
| 0.1 | tree nuts group | |||||
| 25 | transgenic aspirated grain fractions | |||||
| 4 | transgenic corn, field, forage | |||||
| 0.2 | transgenic corn, field, grain | |||||
| 6 | transgenic corn, field, stover | |||||
| 5 | transgenic soybean hulls | |||||
| 2 | transgenic soybeans | |||||
| zinc phosphide (rodenticide) | 11/15/99 page 61788 | 0.05 | potatoes | Yes | Extension | 12-31-01 |
| 0.05 | sugarbeet roots | |||||
| 0.1 | sugarbeet tops | |||||
| Comment: EPA is extending this time-limited tolerance in response to it again granting a Section 18 exemption for the use of zinc phosphide for rodent control in Idaho sugarbeets and potatoes. | ||||||
| clopyralid (herbicide) | 11/17/99 page 62588 | 0.5 | flax seed | Yes | New | 12-31-01 |
| Comment: This time-limited tolerance is being issued in response to a crisis exemption being granted for the use of clopyralid to control thistle in North Dakota flax. | ||||||
| paraquat (herbicide) | 11/22/99 page 63714 | 0.05 | artichokes | Yes | New | 12-31-00 |
| Comment: This time-limited tolerance is being established in response to EPA granting a Section 18 emergency exemption for the use of paraquat to control weeds in California artichokes. | ||||||
| HOE-107892 (and metabolites 113225, 109453, & 094270) (herbicide safener) | 11/22/99 page 63711 | 0.05 | barley grain | Yes | Extension | 12-31-01 |
| 0.5 | barley hay | |||||
| 0.1 | barley straw | |||||
| 1 | pearled barley | |||||
| 0.4 | barley bran | |||||
| 0.1 | barley flour | |||||
| 0.01 | wheat grain | |||||
| 0.05 | wheat straw | |||||
| Comment: This time-limited tolerance is being extended in response to EPA granting a Section 18 emergency exemption for the use of this chemical on wheat and barley. | ||||||
W.S.U. Pullman Home Page Comments and questions: cdaniels@tricity.wsu.edu Technical Assistance: scoates@tricity.wsu.edu Copyright © Washington State University / Disclaimer Electronic Publishing and Appropriate Use Policy University Information: 509/335-3564