





      |
|

|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||
|
|
||
| Compound, Form if Specified) |
|
|
| Sodium Arsenite, As(III) solution |
|
|
| Sodium Arsenite, As (III) dry powder |
|
|
| Sodium Arsenite, As(III) solution |
|
|
| Monomethylarsonic Acid, MMA |
|
|
| Dimethylarsinic Acid, DMA |
|
|
A "safe" level of exposure is difficult to define for inorganic As because of the constant daily exposure to very low levels and the wide range of effects attributed to variable levels of atypically high doses. The most sensitive body system (in terms of exhibiting symptoms) seems to be the skin. Skin disorders have been associated with As in drinking water at 0.01 mg/kg/day for prolonged periods (months to years) (23). This exposure level would translate to a 700 microgram (µg) daily dose in a 70-kg male or a 70-µg dose in a 10-kg two-year-old child.
The doses associated with As intoxication tell us little about hazards without a knowledge of exposure levels. For the next step in deciding whether treated wood might cause harmful health effects, we have to look at whether direct or indirect contact with the wood could substantially increase our daily exposures (over and above what we already receive in our diet and water) to levels that are known to cause the various forms of arsenic toxicity.
With the U.S. Environmental Protection Agency's (EPA's) pre-Bush-administration rule to lower the drinking water standard for As and the political flak over President Bush's decision to reverse the changes, one would think that we are highly exposed to arsenic in drinking water. In fact, the vast majority of people in the U.S. are exposed to levels less than 10 µg/L (10). An adult drinking two liters of water each day would add 20 µg to her daily As intake.
An analysis of the U.S. Food and Drug Administration's Total Diet Study database for the years 1986-1991 showed that an adult's average daily dietary exposure to As is less than 30 µg (13). In the late 1970s, this value may have averaged 60 µg per day (31). Thus, if water consumption and the most recent value for dietary exposure are added, a typical adult may be exposed to 50 µg of naturally occurring arsenic per day. Because a young child (i.e., a two-year old) drinks less water (~1 liter of water per day) and eats less food, a child's daily exposure may be estimated as 18 µg per day (~8 µg from the diet) (13).
The next question is how much more arsenic might we be exposed to if we come in contact with CCA-treated wood? Several exposure scenarios come to mind. If CCA-treated wood is used to build a deck, how much As will someone be exposed to if he rubs his hand across a railing? Will As leach out of the deck during rainfall and contaminate the soil underneath? If CCA-treated wood is used to line garden beds, will As leach out and contaminate the plants? I am not going to address the scenario of a carpenter working with the wood; this constitutes an occupational exposure and the worker should always use a dust mask and wash immediately after handling the wood.
Only a handful of studies have addressed the questions pertinent to consumers. These studies, however, share two common observations: (a) measurable amounts of As leach from CCA-treated wood, but (b) the increase in exposure to humans over what they would receive from background (diet and water) levels seems insignificant with respect to levels known to affect health. For example, two separate studies have examined As levels in soil directly underneath decks. In Florida, a study of nine decks showed average levels ranging from 0.48 mg/kg to 79.1 mg/kg (26). In Connecticut, average As in soils below decks ranged from 9 to 139 mg/kg (28). The background concentration of As in the Florida and Connecticut studies averaged 1.53 and 3.7 mg/kg, respectively. Thus, a child playing under the deck who ingested one gram of soil would potentially receive 139 µg of As. However, bear in mind that As is not absorbed efficiently from the gastrointestinal tract when it is in soil (12), so only about 14 µg would enter the bloodstream (~10% of ingested As in the soil). Furthermore, the child would be intermittently exposed to the soil, and As is quickly metabolized and passed from the body in two to four days.
The good news about the studies with decks is that soil samples taken six inches (15 cm) away from the deck perimeter had a reduction in As concentration of 85% (28), suggesting limited horizontal mobility of As, a limitation also indicated by a study of CCA-treated wood bulkheads along the shores of marine environments (30). Under these latter conditions, with constant water bathing the wood, one would predict high mobility of the As. Yet, the leached As drops off to near background levels at distances beyond three feet (one meter).
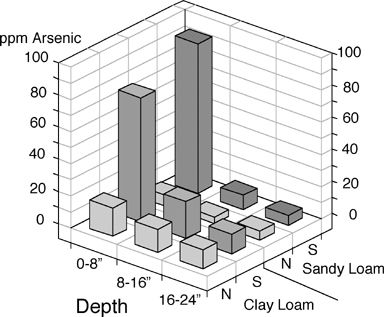
The downward mobility of leached As seems as limited as the horizontal movement. The Florida deck studies showed a rapid decline in As to background levels at an eight-inch depth (26). In another worst-case example, studies with lead arsenate-treated soils during the 1930s showed insignificant leaching below the top eight inches despite high concentrations resulting from repeated yearly spraying of the insecticide (15) (Figure 1).
|
|
|
|
 |
The mobility studies with As, along with studies showing that As can be significantly sorbed to soil (14), indicate that leaching from wood lining gardens would only move a small distance into the soil bed. Furthermore, increases in soil arsenic do not necessarily mean greater uptake by plants. The aforementioned soil sorption propensity also reduces arsenic's bioavailability (14). Studies with grape plants using CCA-treated stakes showed no detectable changes in the As content of vines, stems, or fruit (18).
In a worst-case situation (heavy use of lead arsenate spray), the magnitude of As uptake depends on the specific crop (6, 16). Crop uptake studies on lead arsenate-impacted soils of the Pacific Northwest have shown that As levels in carrots, peas, peppers, and tomatoes were not significantly affected by soil levels. However, beets, kale, eggplant, and lettuce seemed more efficient at bioconcentrating As residues (Table 2). Enhanced uptake of As by lettuce also occurred when plants were grown in a container with a block of CCA-treated wood or a sawdust containing 480 mg/kg As (27). However, when sawdust contained only 32 mg/kg As, uptake hardly changed over background levels. Given that the As uptake studies represent worst-case conditions of As contamination throughout the soil or plant growth adjacent to a piece of CCA-treated wood, the probability is very low that CCA-treated wood can impact As levels in garden plants enough to alter dietary intake.
|
|
|||
|
|
|||
|
|
|||
| Carrot |
|
|
|
| Pea |
|
|
|
| Vetch Hay |
|
|
|
|
|
|||
| Eggplant |
|
|
|
| Onion |
|
|
|
| Peas |
|
|
|
| Pepper |
|
|
|
|
|
|||
| Peas |
|
|
|
|
|
|||
| Alfalfa Hay |
|
|
|
| Beets |
|
|
|
| Kale |
|
|
|
| Lettuce |
|
|
|
| Tomato |
|
|
|
CCA-treated wood is also used in playground structures ("playscapes"). It is reasonable to ask whether children playing on such structures will be exposed to harmful levels of arsenic. Two studies suggest that As can be transferred to the hand when a hand is rubbed across CCA-treated wood. In one study, the maximum amount of As removed by a dry hand moving over a new wood surface was 0.03 µg/cm2 (1). When the hand was wet, 0.3 µg/cm2 was removed. Another study used wet absorbent material to simulate a wet hand on a wood playscape and reported that an average of 0.08 µg/cm2 was removed. The total surface area of both hands on a two- to three-year-old-child has been estimated to be 387 cm2 at the 95th percentile (i.e., the hand's surface area is greater than the surface area in 95% of all other kids) (29). The palms and fingers alone could therefore be considered to have a surface area of 193.5 cm2. Thus, under a worst-case condition where both hands would be placed in a child's mouth and all the As was sucked off and swallowed, the exposure could be as high as 15.5 µg (193.5 cm2 x 0.08 µg/cm2). Using a different set of assumptions, the "playscape" study author estimated an exposure of only 2 µg As (27).
The worst-case increase in As exposure of a two-year-old child who plays on a CCA-treated wood structure and eats vegetables from a garden lined with treated wood can be estimated by combining the daily food and dietary intake with the exposure to the hands. The dietary intake could be increased by a factor of 25% just in case the kid likes eggplant or lettuce, although in reality these would be extremely small components of the diet. Thus, the total exposure could be 35.7 µg (18.1 µg from the diet plus an extra 2.1 µg from the garden plus 15.5 µg from the playground). In summary, the use of CCA-treated wood in a child's total environment could double As exposure under extreme circumstances.
What is the likelihood that such a doubling of As exposure in a child would cause harm? For pesticide risk characterization, we normally compare exposure to some reference dose (RfD) that has a very large built-in safety factor. The RfD is defined by the EPA as a daily dose over one's lifetime that is reasonably certain to have no harmful effects of any kind. Arsenic has not been assigned an RfD as an official "safe" level of exposure as is usual for pesticide risk characterization. However, the Provisional Tolerable Daily Intake (PTDI) developed by the United Nations' World Health Organization is used by the FDA to gauge the safety of daily As intake (13). The PTDI for As is 2.1 µg As per kg body weight per day. This represents an estimated daily dose over a lifetime for which there is a reasonable certainty of no adverse effects.
On a body-weight basis, a two-year old playing on treated wood could receive a dose of 3.6 µg/kg (i.e., 35.7 µg As per 10 kg body weight) . While this dose exceeds the PTDI, bear in mind several important factors that mitigate the hazard. First, the playground and garden vegetables exposure are intermittent while the food and water will be long-term daily events. The PTDI was designed conservatively to protect individuals from chronic exposures over a lifetime. For limited timeframes, exposures could exceed the PTDI without any adverse effects. Second, my calculation for ingestion of As from the child's hands did not consider the high likelihood that only a small fraction of the As would even make it into the mouth. If the arsenic remained on the skin only, then the child would be protected by the low efficiency of As dermal absorption. For these reasons, the likelihood of harmful effects from non-occupational exposures to CCA-treated wood are very low. Remember that As is detoxified and cleared from the body rapidly, so there is no buildup in tissues when exposures are low.
In conclusion, intimate contact with CCA-treated wood does not change arsenic exposure sufficiently to cause harm and current dietary and drinking water exposures seem well below levels known to cause harm. So why did EPA desire to lower the drinking water standard by fivefold? Stay tuned for the next essay, in which I will discuss the wonderful ways that mathematics has been used to make a safe drinking water supply even safer.
Dr. Allan Felsot is an Environmental Toxicologist at WSU and frequent contributor to Agrichemical and Environmental News. His office is located on the Tri-Cities campus, where he can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
If You Are Still Worried About Arsenic from CCA-Treated Wood |
The U.S. Environmental Protection Agency (EPA) released a press advisory May 10, 2001, stating the following:
On May 9, 2001, EPA met with representatives of the wood-treatment industry, including manufacturers and retailers, and with representatives of environmental and public interest groups, to discuss the current status of EPA's reassessment of CCA*-treated wood and to evaluate efforts for informing the public about the safe use and handling of pressure-treated wood. Both meetings featured constructive and direct discussion regarding safety information available to consumers about CCA-treated wood. Industry participants committed to submit a proposal to EPA in two weeks for strengthening consumer safety materials. A public meeting will be convened in early June involving EPA and all stakeholders, to further discuss efforts to strengthen consumer safety information related to CCA-treated wood. EPA is currently reviewing all available scientific information to conduct a thorough and comprehensive reassessment of CCA-treated wood. As part of this reassessment, the Agency is expediting a risk assessment, expected to be completed in June, focusing on children's potential exposure from playground equipment constructed with CCA-treated wood. EPA remains committed to ensuring ample opportunity for public involvement in all aspects of this process.
(*CCA = Chromated copper arsenate; see preceding article.)
Hello. It is I again, the Queen Bee of
Labels (QBL). Some of our loyal (royal) readers may have noticed
that it has been awhile since the QBL graced these pages. In
fact, the QBL has not had much to say since she got graphic back
in the December issue of this upstanding newsletter ("The QBL Gets Graphic," AENews
Issue No. 176). The start of 2001
was the beginning of a Registrants' Royal Reprieve. Over the
last few months, the QBL has shown restraint and has refrained
from assisting registrants by pointing out examples of their
lousy pesticide labels. During said Reprieve, no Non-Anom Awards
(see AENews Issue No. 171, July 2000)
have been handed out; no registrants have been called on the
carpet to atone for their misdeeds. 
The QBL thought it both refreshing and useful to grant a Royal Time Out. Envisioning a period during which each registrant examined its own soul and reflected on its various wayward pesticide labels, the QBL hoped that the registrants would take the opportunity to ponder (and execute) some positive changes. Moreover, Her Royal Highness (HRH) thought perhaps this Reprieve period would give the U.S. Environmental Protection Agency (EPA, the governmental entity ostensibly in charge of reviewing pesticide labels) time to do the right thing and simply appoint HRH to her rightful position as the Queen Bee of Labels, thereby making all things right (see "If I Were The Queen of Labels," AENews Issue No. 169, May 2000).
Well, the QBL hopes that you have enjoyed this Reprieve because it has now come to an end. Sad to say, despite the Time Out, EPA has not been forthcoming with the job offer, registrants did no (or insufficient) soul searching, and things in the world of pesticide labels are still a mess.
The termination of the Reprieve was brought about by an outstanding example of this mess: Rohm & Haas' Confirm T/O label. Indeed, this label caused the QBL to have the following Royal Revelation: T/O does not stand for (or in any way imply use on) Turf and Ornamentals. (That said it occurs to the QBL that perhaps one should not admit to having Revelations because it does seem to indicate a certain lack of omniscience.)
Prior to this Revelation, which really rocked the royal socks, the QBL had been under the mistaken impression that when a registrant included T/O in a product name that this stood for Turf and Ornamental. What? You thought so, too? Consider yourself in not only good, but royal, well-bred, soft-spoken, kind, thrifty, cheerful, and lofty company. But there you have it: the QBL is once again spilling the beans, revealing a previously unknown "truth" about pesticide labels: "T/O" is not synonymous with "Turf/Ornamentals."
The Confirm T/O label initially came to the attention of the QBL through a problem unrelated to either its T or its O.
The Royal wRath was drawn by a notation by Rohm & Haas on the cover sheet for the revised label (ooh and don't get the QBL started on the whole revision process--see AENews Issue No. 173, September 2000) stating that mint uses had been added to the label. Because the trusty staff at Washington State University's Pesticide Information Center (PIC) actually read pesticide labels, and because, when doing so, they could not find any directions for mint on the label, the QBL herself called Rohm & Haas. It should be noted here, in the event that it is not obvious, that when a pesticide label causes such confusion that the QBL must pick up the phone and make a call for clarification, all things are not coming up roses for the registrant in question. After several false starts, where the QBL was informed that of course mint use directions were included on the label, it finally came to light that the label cover was in error and that Confirm T/O was in fact not labeled for use on mint.
Then came the Royal Revelation (T/O does not equal Turf and Ornamentals). While cooling the royal heels on "hold" as Rohm & Haas searched in vain for the word "mint," HRH the QBL noticed that the Confirm T/O label contains use directions for ornamentals, bushberries, caneberries, cole crops, leafy vegetables and turnips, fruiting vegetables, pome fruits, pecans, and walnuts; any directions for turf use were conspicuously absent. Not one to be put off by dealing with a wayward registrant, after resolving the Mint Issue, the QBL went on to ask why was the label designated as Turf and Ornamental when it clearly was intended for other crops? HRH was informed that the T/O designation was sometimes used by registrants to designate products packaged in smaller container sizes that are also intended for homeowner use. Whereupon the QBL reviewed the facts. Indeed, Confirm T/O is a one-quart package. However, the directions for all uses except ornamentals are given in per-acre rates; only the QBL's more ambitious friends tend multi-acre home gardens. The Rohm & Haas representative agreed that this was problematic. Reading in more detail, the application directions for cole crops, leafy vegetables and turnips, fruiting vegetables, pome fruits, pecans, and walnuts all discuss using conventional ground equipment while the application directions for the berries states "Make applications by conventional boom or air-blast sprayers." While the QBL does acknowledge that there are directions for using a hand sprayer to treat ornamentals, she does not consider application by air-blast sprayers to be one for home and garden use.
In days of yore, when T/O still stood for Turf and Ornamental, why might Rohm & Haas have called this product Confirm T/O? As turf uses are not included on the label, why not just call the product Confirm-O? After all, it has a nice ring to it and a good beat. But in truth, while Confirm-O is closer to the truth, the QBL can see that, sounding a lot like Wham-O or Blam-O, this name might not be such a hot idea. All right, if Confirm-O was not really an option, how about Confirm O Plus to indicate a product for use on Ornamentals "and other stuff?" If Rohm & Haas decides it can't live with that, then the QBL suggests Confirm Misc as a viable alternate.
But then back to the Royal Revelation that T/O is not synonymous with Turf and Ornamentals. If not Turf and Ornamental, then what does T/O mean as part of a pesticide product name? In the case of Rohm & Haas, it seems apparent that T/O indicates crops not otherwise wanted on another label. Some suggested answers are:
This
Stuff and Other Stuff
Truth Oscillations
Terminally Obsolete
Tired Odds & Ends
Twaddle Only
Terminology Orphans
After pondering this weighty issue for quite long enough, the QBL believes that T/O really stands for Token Offerings. If you think that you have a better idea, please feel free to let the QBL know but be forewarned: this is Monarchy. While you are free to speak, you may not be heard.
Once the Queen Bee of Labels is appointed to her rightful place at EPA, Rohm & Haas might want to reconsider their whole approach to pesticide labeling. The QBL has a long memory and it will be years before the Confirm-O indiscretion is forgotten.
Jane M. Thomas reigns from WSU's Pesticide Information Center, where she bides her time as Pesticide Notification Network Coordinator until her Phone Call from EPA comes. Until that time, she can be reached on a regular telephone at (509) 372-7493 or on common e-mail at jmthomas@tricity.wsu.edu.
For more QBL observations and scathing
commentaries, see AENews Issues No. 169,
171,
172,
173,
175,
and 176.
Issue dates are May,
July,
August,
September,
November,
and December,
2000, respectively.
Last month, thrips were presented as the AENews Pest of the Month. This month, I will detail a pair of field studies involving thrips in dry bulb onions. I undertook these studies in the summer and fall of 2000 in response to complaints from dry bulb onion growers that they can't control thrips with pyrethroid and organophosphate insecticides.
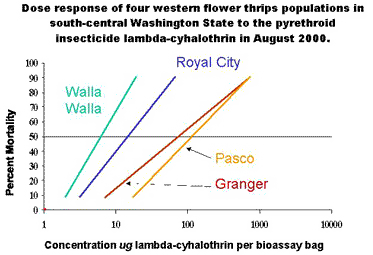
In August 2000, we surveyed thrips populations in four separate onion fields in Central Washington (near Royal City, Granger, Pasco, and Walla Walla). The onion field in Granger had been treated four times with lambda-cyhalothrin. The field in Royal City had been treated once with cypermethrin, twice with lambda-cyhalothrin and once with methyl-parathion. The field in Pasco had been treated twice with lambda-cyhalothrin and the field in Walla Walla had been treated once with lambda-cyhalothrin. No onion thrips were present in the onion fields near Royal City, Pasco, and Granger; only western flower thrips were found in those fields. The only site in the study that contained both thrips species was the Walla Walla field.
Suspecting the thrips might be developing pyrethroid tolerance, we conducted tolerance tests. Levels of lambda-cyhalothrin tolerance were high in Pasco, Granger, and Royal City. There was substantial variation in the dose response in Walla Walla; some of the individuals in that thrips population remained susceptible to lambda-cyhalothrin (Figure 1).
|
|
 |
These findings led me to speculate that western flower thrips were replacing onion thrips over the course of the season in onion fields after the fields were treated with pyrethroid insecticides. Pyrethroid insecticides are more lethal to onion thrips than they are to western flower thrips, so the latter became predominant.
Thrips have the potential to remain problematic beyond the field. Do thrips remain in the onion bulbs through harvest? Do they continue to persist in stored onion? These are critical questions for onion growers. The sites where thrips feed on the onions may create entry points for such storage diseases as botrytis gray mold, Penicillum, and aspergillus.
To help answer these questions, I treated a small plot of onions in a commercial onion field near Royal City in early September 2000 with chlorfenapyr. Chlorfenapyr is a non-registered anti-metabolite insecticide that proved to be a very effective thrips control compound in work I did on strawberries in California several years ago. In addition to my chlorfenapyr treatment, this entire onion field had been treated twice with lambda-cyhalothrin. In early October 2000, we harvested the chlorfenapyr-treated plot as well as an equivalent number of commercially treated onions from a nearby section of the field.
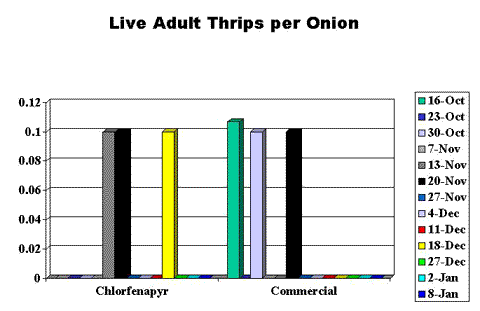
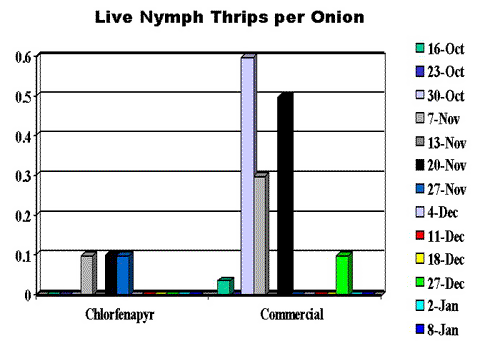
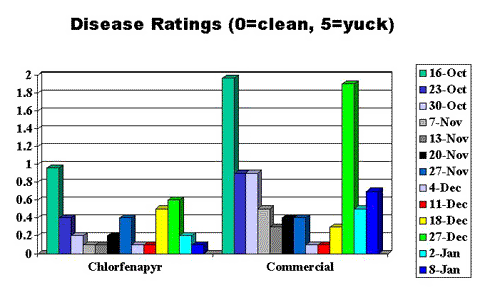
The onions were held in storage chambers at Washington State University Prosser; the chambers were climate-controlled to between 47° and 50°F and 65% relative humidity. We surveyed sub-samples of ten onions from the chlorfenapyr-treated plot and the commercial plot each week throughout the fall and into early winter 2001, counting and recording the presence of live adult or nymph thrips (Figures 2 and 3, end of article) and observing and recording the incidence and severity of disease (Figure 4, end of article).
Adult thrips populations were low in the stored onions: around 0.1 thrip per onion (Figure 2). However, it was surprising to observe live adult thrips after two months of storage. Live nymph thrips populations were substantially higher in the commercially treated onions at between 0.6 and 0.3 nymph per onion compared to about 0.1 thrip per onion in the chlorfenapyr-treated onions for the first several weeks after the onions were placed in storage (Figure 3). No live adult or nymph thrips were observed after mid-December.
Disease ratings consisted of evaluating the "necks" of the onions for the presence of orange (aspergillus) or gray mold (botrytis). On the Brophy Onion Disease Scale (0 to 5, 0 = no visible disease present and 5 = "yuck" or inedible), disease never surpassed a 2. However there was a definite trend toward an increased incidence of disease in the commercially treated onions compared to the chlorfenapyr-treated onions.
The results of these field surveys indicate that the chlorfenapyr treatment was effective in suppressing thrips populations.
As registration for chlorfenapyr in onion is not being sought, research on other effective chemicals is needed. We will be expanding our studies on thrips in dry bulb onions with grants I have received from the Washington State Commission on Pesticide Registration, the Pacific Northwest Vegetable Association, and the Columbia Basin Vegetable Seed Association. I thank Gary Pelter, (WSU Cooperative Extension, Grant County), Ron Wight and Melinda Brophy (WSU Prosser) and Dr. William Brindley (Utah State University) for their assistance with these studies.
|
|
 |
|
|
 |
|
|
 |
The fifth meeting of the Food and Environmental Quality Laboratory (FEQL) Advisory Board was held on the Washington State University (WSU) Tri-Cities campus April 17, 2001. Eleven board members were present, along with six representatives of FEQL/WSU.
With board chair Scott McKinnie's term ending June 30, 2001, the first order of business was election of officers. As chair-elect, I accepted the nomination for board chair for the coming year (July 2001 through June 2002) and was elected. Board member Don Abbott from the Washington State Department of Ecology was elected vice-chair/chair-elect.
Board member positions, each representing a different aspect of Pacific Northwest agriculture, food safety, and environmental quality, are mandated by the Washington State legislation that created the FEQL and its board. Two of those positions, one representing farm labor and one representing food processing, will become vacant June 30, 2001. The FEQL board is accepting suggestions for qualified individuals who can fill those positions. Parties wishing to recommend a farm labor or food processing representative can contact me or any other member of the FEQL staff or advisory board until July 1, 2001.
(Other board member positions and their current representatives are as follows: health care professional knowledgeable in worker exposure to pesticides, Matt Keifer, Pacific Northwest Agriculture Safety and Health Center; WSU research administrator, James Zuiches (Dean of the College of Agriculture and Home Economics); Washington State Department of Agriculture, Royal Schoen; Washington State Department of Ecology, Don Abbott; Washington State Department of Health, Barbara Morrissey; Washington State Department of Labor and Industry, Janet Kurina; Washington State Commission on Pesticide Registration, Ann George; privately owned Washington State analytical laboratory, John Peterson, Englar Food Laboratories; federal regional pesticide laboratory, Rick Long, US Food and Drug Administration; an Idaho laboratory, Gregg Möller, University of Idaho; an Oregon laboratory, Jeff Jenkins (to be replaced by Kim Anderson after June 30, 2001), Oregon State University; chemical/fertilizer industry representative, Scott McKinnie, Far West Agribusiness Association; farm organization, Dave Winckler, Ironwood Orchard; marketer, Wally Ewart, Northwest Horticultural Council; environmental organization, Peggy Adams, Palouse-Clearwater Environmental Institute. As a representative of the League of Women Voters, I hold the consumer position.)
In the wake of a state budget cut scare that occurred in late March, the board discussed the need to communicate the value of the FEQL to legislators and other audiences, including the agricultural community, the general public, and the university itself. These stakeholders need to understand the important work that the FEQL is doing. Allan Felsot submitted a statement that describes the FEQL and its impact on our state:
The FEQL was mandated by the Washington State Legislature to focus research and extension efforts on all aspects of crop protection technologies across the state. The need for such a WSU facility grew out of two concerns: potentially devastating losses of crop protection tools for the minor crops characteristic of Washington agriculture and the safety of these tools to human health and the environment. The FEQL faculty have developed a productive program over the last eight years to readily address these divergent issues. The programs are valued by the general public, which can directly obtain information from the FEQL about the use and safety of all types of pest control technologies used in rural and urban environments. The programs are also valued by the agricultural industry for providing objective and critical analyses of current environmental regulations and issues, as well as conducting applied research on best management practices that help make agriculture even safer. For example, the FEQL studies pesticide drift and solutions for management and resolution of conflicts. FEQL faculty have been studying application of crop production agents through drip irrigation systems that are extremely critical to the Yakima Valley for improving water use efficiency. In cooperation with the WSDA and the ODA, the FEQL faculty maintain a publicly accessible Internet database on all pesticide registrations in Washington and Oregon. Literally tens of thousands of Washington residents are reached each year through the extension services of the FEQL. FEQL faculty teach courses in environmental chemistry and toxicology, subjects that are extremely important to the promulgation of science-based human health and environmental legislation. In short, the philosophy of the FEQL program is to provide information and innovative solutions to citizens of Washington State.
Board members and FEQL staff discussed ways to disseminate the message that FEQL is doing good things for Washington State, including offering tours of the lab, mentioning FEQL and its projects during public speaking opportunities, and making Agrichemical and Environmental News available to a wider audience.
FEQL faculty members Doug Walsh, Vince Hebert, Allan Felsot, and Catherine Daniels gave presentations on the status of their respective programs. Dr. Walsh discussed his entomological field work and presented a list of projects, publications, and speaking engagements. Dr. Hebert explained the lab's progress toward Good Laboratory Practices (GLP) certification and discussed his various projects, including "Maintenance of Guthion and Sevin Registrations on Pome Fruits" underway with Dr. Felsot. Dr. Felsot described his current teaching, extension, and research activities, including a recent project "Assessing the Safety of Herbicides for Vegetation Management in the Missoula Valley Region." Dr. Daniels presented the status of her programs, including the Pesticide Information Center (PIC) and its various activities: the Agrichemical and Environmental News, the Pesticide Information Center On-Line (PICOL) database (http://picol.cahe.wsu.edu), and the Pesticide Notification Network (PNN, http://www.pnn.wsu.edu). She detailed projects underway in response to mandates from federal 406 funding, all of which need to be completed by September 1, 2001: a new pest management web page, eight crop profiles, and initiation of a pest management strategic plan.
The board also heard a presentation from Washington Association of Wheat Growers' Gretchen Borck about recent issues and controversies between environmental groups and wheat farmers over residue (wheat stubble) burning. A Spokane-based environmental group called Save Our Summers (SOS) is alleging harm from farming practices that may be subject to interpretation via the Americans with Disabilities Act, while wheat growers maintain that burning is a well-conceived best management practice tool that works in concert with no-till production systems to safeguard land from erosion and a host of other problems.
The FEQL Advisory Board will meet again in November 2001.
Marilyn Perkins is a member of the League of Women Voters of Washington and Chair of the FEQL Advisory Board. She can be reached at (509) 783-8610 or at perkinsjohn@msn.com.
Washington State University's College of
Agriculture and Home Economics (WSU CAHE) recognized outstanding
contributions to teaching, research, and extension activities
at its 42nd Annual Awards Banquet and Program, April 21st, 2001.
Faculty members recognized included Dr. William Johnston, Associate
Professor of Crop and Soil Sciences; Dr. Richard Zack, Assistant
Professor of Entomology; Dr. Markus Flury, Assistant Professor
of Crop and Soil Sciences; Dr. Barry Swanson, Professor of Food
Science and Human Nutrition; and Dr. Allan Felsot, Environmental
Toxicologist at the WSU Food and Environmental Quality Laboratory.
Dr. Johnston received the college Alumni Association Undergraduate Advising Award. He has been a member of the WSU faculty for twenty years. He teaches turf and forage classes, conducts workshops, and coordinates the Crop and Soil Sciences Department's internship program. He also serves as chair of his department's scholarship committee, as a member of the department's undergraduate curriculum committee, and advisor of the student Turf Club. He has advised an average of twenty-eight undergraduate and graduate students the past four years.
Dr. Zack received the R.M. Wade Excellence in Teaching Award. Zack has been at WSU for twenty years. He was instrumental in increasing the enrollment of Entomology 101 from twenty-three students when he began teaching the class in 1997 to more than ninety in 2000. Altogether, his six classes account for almost half of his department's undergraduate enrollment. Zack practices his delivery the night before each class he teaches. The result is a lecture that appears to be extemporaneous, according to John Brown, department chair. "Students repeatedly identify Dr. Zack as the best instructor they have had at WSU."
Drs. Flury and Swanson were named co-winners of the Faculty in Research Award. Flury has been at WSU for four years. His research spans the field of soil physics, from the transport of viruses, radio nuclides and dyes to the development of novel means of determining fundamental physical properties of soils. Swanson has been with WSU since 1974. He has an international reputation for research on grain legumes, sugar-fatty acid polyesters for fat substitutes, non-thermal food processing, and food safety.
Dr. Felsot, a tireless ambassador of agricultural science, received the Extension Faculty Excellence Award. Dr. Felsot averaged fifty presentations a year the past two years, on topics ranging from biotechnology and transgenic crops to salmon, water quality, and pesticides. He has been with WSU for eight years. As readers of AENews know, Felsot tackles tough issues. He has worked with disputing growers in Badger Canyon and the Horse Heaven Hills over long-standing allegations of pesticide drift. Tests he conducted found that direct drift from the Horse Heavens was not causing herbicide injury to crops.
AENews editorial staff thanks the WSU CAHE Information Department for their assistance with these announcements and congratulates these deserving award winners.

The Lygus bug, Lygus hesperus, is native to the Pacific Northwest. It is known to be an agronomic insect pest of many crops, including apples, apricots, caneberries, dry beans, forage (alfalfa) seeds, lentils, lima beans, pears, plums, prunes, potatoes, snap beans, spinach, strawberries, sugar beets, and several vegetable seed crops (1).
Lygus are "true bugs." They have piercing, sucking mouthparts in the form of slender, segmented beaks that arise from the front of their heads and extend back along the ventral (lower) sides of their bodies.
Lygus feeding has been likened to chemical injury. The bugs insert their mouthparts into plant tissue and inject digestive enzymes. After probing and injecting for awhile, Lygus return to areas they previously probed and ingest the partially digested plant tissues. Lygus feeding damage can vary from crop to crop. In cotton, Lygus feeding can cause flower bud abortion, death of plant terminals, and staining of lint. In fruit and pod vegetable crops, feeding results in cosmetic quality loss. In seed crops, Lygus feeding often results in a reduction of seed set.
Lygus in the Pacific Northwest have an extensive host range of both introduced and exotic plants and weeds including burclover, Canada thistle, chickweed, common groundsel, curly dock, filaree, horseweed, kochia, knotweed, lambsquarters, lupine, mullein, mustards, pepperweed, pigweed, pineappleweed, rabbit brush, ragweed, redmaids, Russian thistle, sage, shepherd's purse, smart weed, smotherweed, sweet clover, and wild radish.
Lygus are highly mobile. They are strong flyers and have been documented to fly as far as thirty miles in cotton-growing regions of California.
Lygus overwinter as adults in plants and plant debris, becoming active as temperatures warm in spring. They mate soon after emerging; mated females begin laying eggs several days later. Lygus develop through five nymphal instars (larval stages) before emerging as adults.
Entomologists in California developed a model correlating climate to biological changes (known as a "phenology model") that proved effective at predicting the timing of Lygus generation cycles (3). This model has been tested in eastern Washington and has proven relatively accurate at predicting the first-generation hatch of Lygus in spring. However, the model is less accurate as spring progresses (2). Lygus bug populations typically will complete three generations per year in eastern Washington, eastern Oregon, and western Idaho.
Organophosphate, carbamate, and pyrethroid insecticides have all been used extensively to suppress Lygus populations across a wide range of crops. Dan Mayer (WSU) and Bill Brindley (Utah State) have documented insecticide resistance in Lygus populations infesting alfalfa and vegetable seed crops. Substantial efforts have been made at developing biological control programs for Lygus. Unfortunately, these programs do not generally reduce Lygus populations below economically damaging levels on many high-value crops like alfalfa seed.
Dr. Doug Walsh is an Extension Entomologist with WSU. He can be reached at (509) 786-2226 or dwalsh@tricity.wsu.edu.
Washington Pest Consultants Association (WaPCA) has been involved in recycling plastic pesticide containers since the early 1990s. They organize an annual series of collection dates and sites, contracting with Northwest Ag Plastics to collect and granulate the plastic containers. A schedule for eastern and western Washington dates and times through October is available on-line at
There is no charge for this important service. Contact information, container clean-up criteria, and other details are posted at the URL above.
|
|
The Pesticide Notification Network (PNN) is operated by WSU's Pesticide Information Center for the Washington State Commission on Pesticide Registration. The system is designed to distribute pesticide registration and label change information to groups representing Washington's pesticide users. PNN notifications are now available on our web page. To review those sent out in the month two months prior to this issue's date, either access the PNN page via the Pesticide Information Center On-Line (PICOL) Main Page on URL http://picol.cahe.wsu.edu/ or directly via URL http://www.pnn.wsu.edu. We hope that this new electronic format will be useful. Please let us know what you think by submitting comments via e-mail to Jane Thomas at jmthomas@tricity.wsu.edu.
| Chemical (type) | Federal Register | Tolerance (ppm) | Commodity (raw) |
|
||
| Yes/No | New/Extension | Expiration Date | ||||
| ethametsulfuron methyl (herbicide) | 4/6/01 pg. 18201 | 0.02 | canola | No | N/A | N/A |
| 0.02 | crambe | |||||
| 0.02 | rapeseed | |||||
| fenpyroximate (insecticide) | 4/10/01 pg. 18561 | 1 | wine grapes | Yes | New | 4/12/04 |
| 10 | hops | |||||
| Comment: This time-limited tolerance was requested as an import tolerance by Nihon Nohyaku. | ||||||
| imidacloprid (insecticide) | 4/10/01 pg. 18554 | 3.5 | cilantro | No | N/A | N/A |
| 0.1 | sweet corn, forage | |||||
| 0.2 | sweet corn, stover | |||||
| 0.05 | sweet corn (K+CWHR) | |||||
| 0.2 | field corn, fodder | |||||
| 0.1 | field corn, forage | |||||
| 0.05 | field corn, grain | |||||
| 1 | edible podded beans | |||||
| 1 | succulent shelled beans | |||||
| 3.5 | turnip greens | |||||
| 6 | leaf petiole vegetable subgroup | |||||
| zoxamide (fungicide) | 4/11/01 pg. 18725 | 0.06 | potato, tuber | No | N/A | N/A |
| 0.3 | potato, granule/flake | |||||
| 0.1 | potato, wet peel | |||||
| 3 | grape | |||||
| 15 | grape, raisins | |||||
| propiconazole (fungicide) | 4/18/01 pg. 19863 | 12 | field corn, stover | Yes | New | 3/30/04 |
| 12 | field corn, forage | |||||
| 0.1 | field corn, grain | |||||
| 0.1 | sweet corn (K+CWHR) | |||||
| Comment: With this action EPA is reestablishing tolerances that expired 12/31/00. | ||||||
| metolachlor (herbicide) | 4/18/01 pg. 19860 | 0.1 | tomatoes | Yes | Extension | 6/30/02 |
| 0.3 | tomato puree | |||||
| 0.6 | tomato paste | |||||
| Comment: These time-limited tolerances are being extended due to EPA again granting Section 18 exemptions for the use of metolachlor on tomatoes for control of weeds in Ohio, Pennsylvania, Michigan, Maryland, California, and Virginia. | ||||||
| hexythiazox (ovicide/miticide) | 4/18/01 pg. 19879 | 1 | caneberry crop subgroup | No | N/A | N/A |
| 0.3 | nut, tree, group | |||||
| 2 | peppermint, tops | |||||
| 0.4 | plum, prune, dried | |||||
| 0.1 | plum, prune, fresh | |||||
| 2 | spearmint, tops | |||||
W.S.U. Pullman Home Page Comments and questions: cdaniels@tricity.wsu.edu Technical Assistance: scoates@tricity.wsu.edu Copyright © Washington State University / Disclaimer Electronic Publishing and Appropriate Use Policy University Information: 509/335-3564