
October 2002, Issue No. 198
A monthly report on environmental
and pesticide related issues
In This Issue
NOTE
ABOUT PRINTING A HARD COPY
Some AENews feature articles are available
in Portable Document Format (PDF) version, which is recommended for
printing. If you do not have Adobe
Acrobat Reader (required to read PDF files), this free program is
available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
If you choose to print from the HTML version you currently see on
your screen, you may need to set your browser's printing preferences
so that no margins are cropped.
|
||
| |
||
NOTE
ABOUT PRINTING A HARD COPY
Some AENews feature articles are available
in Portable Document Format (PDF) version, which is recommended for
printing. If you do not have Adobe
Acrobat Reader (required to read PDF files), this free program is
available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
If you choose to print from the HTML version you currently see on
your screen, you may need to set your browser's printing preferences
so that no margins are cropped.
This issue has been viewed
times since October 4, 2002.
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
What’s Cooking with Vinegar Recommendations?
Acetic Acid as Herbicide
Dr. Catherine H. Daniels, Pesticide Coordinator, WSU
In May 2002, U.S. Department of Agriculture’s Agricultural Research Service (USDA-ARS) issued a press release describing their research on weed control using vinegar. The research was prompted by the organic farming community's need for an inexpensive and environmentally benign weed killer. Greenhouse and field studies indicated that while 5% vinegar solutions did not produce reliable weed control, solutions of 10, 15, and 20% provided 80-100% control of certain annual weeds (foxtail, lambsquarters, pigweed, and velvetleaf). Perennial weeds (Canada thistle) treated with 5% vinegar showed 100% shoot burndown but roots were not affected, therefore shoots always re-grew. Study details can be found at http://www.barc.usda.gov/anri/sasl/vinegar.html. The press release noted the potential use of vinegar as an ideal sidewalk crack and crevice treatment. Homeowners around the Pacific Northwest had already heard about purported vinegar uses for killing blackberries in a June 6, 2001, Seattle Post Intelligencer article and had deluged Cooperative Extension offices and Master Gardeners for more information. (See also "Acetic Acid: Miracle Herbicide? Sour Product Promises Sweet Results," AENews Issue No. 185, September 2001). There is something appealing about the idea of a commonly available, inexpensive material such as household vinegar being effective against weeds. It does not harm people, in fact people consume it every day, yet it is deadly to our mortal enemies: lawn weeds. Why, such is the stuff of dreams in the pesticide issues arena!
From Dreams to Reality
Well, I hate to be one who breaks the bad news, but we’re not talking about household vinegar here. The typical strength of the stuff we toss with olive oil or run through the cleaning cycle on our coffee makers is 5% acetic acid, a concentration shown to be less-than-reliable by the ARS study. Beyond that sad fact, responsible stewardship requires that those of us in the business of making pesticide recommendations ask certain questions before embracing a pest control technology. The first of those questions should be, “What products containing this ingredient are registered for use?”
Five herbicide products are currently registered in Washington State that contain acetic acid. Two of them come as 25% concentrates with instructions to dilute down to 6.25% and use on rights-of-ways, non-crop, and industrial lands (St. Gabriel Labs Burn Out Weed and Grass Killer Concentrate, Nature's Glory Weed and Grass Killer Concentrate). While these may be useful to commercial applicators, they are not labeled for home uses. Three products are labeled for homeowner use (St. Gabriel Labs’ Fast Acting Burn Out RTU, Nature's Glory Weed and Grass Killer RTU, Greenergy's Blackberry and Brush Block). Their acetic acid concentrations are 6.25%, 6.25%, and 7% respectively. Curiously, Greenergy's product label lists acetic acid as an inert ingredient; citric acid is listed as the active ingredient. By listing the ingredients this way, Greenergy is able to take advantage of EPA's "Minimum Risk Pesticide" definition. Products falling under this category are also known as “25(b) products” after the FIFRA rule describing criteria for minimum risk pesticides. Such products need not be registered at the Federal level and do not carry an EPA registration number. However, Washington law requires that 25(b) products go through the Washington State Department of Agriculture's (WSDA) registration process regardless. Oregon law allows the Oregon Department of Agriculture (ODA) to follow EPA's lead on 25(b)s, so Greenergy does not have to register Blackberry and Brush Block in Oregon. Fast Acting Burn Out RTU is not registered in Oregon, leaving Nature's Glory Weed and Grass Killer RTU and Blackberry and Brush Block legal for use in Oregon.
Down to Earth With a Thump
Another question we must pose if we are to make responsible pesticide recommendations is whether the active ingredient in question works under environmental conditions found in our region. Preliminary field tests in Washington State using 7% vinegar solutions showed results similar to the ARS study at 5%, namely lack of reliable weed control. While extension personnel in Washington and Oregon are able to legally recommend any of the homeowner-registered products listed above (three in Washington, two in Oregon), the data demonstrates erratic weed control. In other words, people should be told that if they want to use vinegar at the registered concentrations it might not work in their situation.
Turning Up the Juice
A few weeks ago a product called Bradfield Horticultural Vinegar (20% acetic acid), sold by Bradfield Industries, was found in a Washington home and garden center. At first glance it seemed the answer consumers had been clamoring for. Upon closer examination however, the product is not registered with EPA and does not qualify under the Minimum Risk Pesticide category for non-registration. The Colorado Department of Agriculture went so far as to issue a media release, warning consumers about the unregistered product (http://www.ag.state.co.us/commissioner/press/2002/Vinegar.html.) Apparently the company is trying to take advantage of a gray area of the legal system. There is a part of federal law which states that if a product clearly has uses other than as a pesticide AND the company makes no claims about that product having pesticidal uses, it does not have to be registered as a pesticide. This law makes sense for things like citric acid, culinary herbs and their oils, and other products that are used in a wide range of applications besides pesticides. Acetic acid has numerous other uses so it, too, falls under this category.
Bradfield Industries tried to market their product in Oregon with herbicide uses listed on the label. Oregon Department of Agriculture inspectors contacted Bradfield and told them that if they made pesticide claims on their label, it would have to be registered as a pesticide in Oregon. The company subsequently changed their label. The Bradfield jugs found at the Washington home and garden center bore a plain label stating the product’s name and acetic acid percentage only. But here’s the kicker: attached to the jug handles by a twist-tie were information sheets discussing some of the common uses for acetic acid: cleaning farm equipment, lowering pH in fertigation and other foliar sprays, AND AS A HERBICIDE! These attached sheets also state that "since vinegar is on the EPA Generally Recognized as Safe (GRAS) List, registration is unnecessary." That statement is misleading and only partially true. Acetic acid is on the list of GRAS inerts, but it is not on the GRAS active ingredient list (remember our earlier explanation of the Greenergy product?)
When we put a call into the Colorado Department of Agriculture to ask them about their concerns, we were referred to Judith Sturgess, EPA Region VII, who is handling an EPA action against Bradfield Industries. This action involves a stop sale order as well as a civil complaint with a financial penalty. EPA's position, according to Ms. Sturgess, is that the vinegar product is illegal as it is not registered as a pesticide, is not in the registration queue, and is not eligible for Minimum Risk status, yet is being distributed with information describing a pesticide use. EPA bases their position on information sheets found with the product and also on information from the Bradfield Web site at http://www.bradfieldind.com/, which continues to describe the product as a herbicide.
In A Pickle
Let’s see where this series of events has brought us. USDA-ARS has done research to show that 20% concentrations of acetic acid work to control weeds. Many people are clamoring for access to products with these high concentrations, yet no company seems willing to go through the EPA registration process with a 20% product. Adding to the problem, it seems that some USDA personnel are locating sources of 20% product (distributors who sell 20% vinegar to food outlets) and recommending them to the public as sources of herbicide. We have received e-mails to this effect and have followed Web threads evidencing such recommendations.
Can those of us in extension take the same short cut and recommend the higher (unregistered) concentrations, or in fact give people lists of distributors? The answer, at least in Washington State, is NO. There is a legal fine point buried in here. If any material claims to kill pests (weeds) it becomes a pesticide, no matter who does the “claiming.” We cannot make a recommendation for an unregistered pesticide. Okay, so what if the material does not claim to be a “pesticide,” it is simply a “food grade 20% solution” (normally) sold to those who make pickles? Can we recommend it then? The answer is again, NO. As Ms. Sturgess pointed out, making lists of 20% vinegar outlets is tantamount to directing people to use an unregistered pesticide.
Some may ask what is the big deal over such picky paperwork details when people are clamoring to use the product? After all, isn't the catchphrase of the marketplace "Let the buyer beware"? Besides being legally culpable in recommending unregistered pesticides, we have practical safety concerns for homeowners. Homeowners are not trained in safe handling and storage of concentrated chemicals. Acetic acid concentrations over 11% can cause burns upon skin contact. Eye contact can result in severe burns and permanent corneal injury. The 25% acetic acid concentrations registered through EPA and the states for commercial use all have restricted entry intervals of 48 hours and list personal protection equipment to be used by the applicator. None of this safety information is included on the twist-tie information on the jug of Bradfield Horticultural Vinegar. Because the public is used to thinking of vinegar as something you can safely splash on your salad and eat they are generally unaware of potential dangers of a higher concentration.
Concentrating on Solutions
The simple solution seems to be for a company to step forward and register the 20% concentration as a herbicide. Judging from the reactions on Web threads and from our own experience after publication of newspaper articles, there is certainly enough demand out there to make it profitable. Research, courtesy of USDA, has already been done. Extension stands ready to recommend registered products. So who will step up to the plate?
Dr. Catherine Daniels is Pesticide Coordinator for WSU and the Director of the Washington State Pest Management Resource Service, http://wsprs.wsu.edu.She can be reached at (509) 372-7495 or cdaniels@tricity.wsu.edu. Information presented in this article is condensed into a two-page fact sheet targeted at county agents available at http://wsprs.wsu.edu/VinegarFactSheet.pdf.
Return to Table of Contents for the October, 2002 issue
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
EPA and FQPA
National Progress and Region 10 Projects
Sandra Halstead, FQPA Specialist, EPA Region 10
August 3, 2002, marked the sixth anniversary of the passage of the Food Quality Protection Act (FQPA), an intensive scientific and regulatory effort to ensure that all registered pesticides meet a tougher food safety standard. Among other provisions, FQPA mandates a single, health-based standard for all pesticides in all foods with special protections for infants and children.
Under FQPA statutory timelines, EPA was required to evaluate over sixty-six percent of existing pesticide tolerances (i.e., acceptable level of pesticide residues) by August 3, 2002. In meeting the deadline, EPA reassessed over 6,400 tolerances, including revoking over 1,900 tolerances for pesticide residues on food. EPA gave priority to the evaluation of specific pesticide classes thought to pose the greatest risk, including the organophosphate, carbamate, and organochlorine classes and potential carcinogens. In addition to meeting the tolerance reassessment goal, EPA completed the full evaluation of four individual pesticides (benomyl, diazinon, endosulfan, and lindane) to comply with a consent agreement with the Natural Resources Defense Council (NRDC).
Aggregate and Cumulative Exposure
As a part of the evaluation process, FQPA required EPA to develop methods for assessing combined (or “aggregate”) exposures from food, water, and residential sources. Amendments to the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) in 1988 require EPA to re-evaluate all pesticides registered prior to 1984, to ensure they meet current safety standards, including ecological and worker risks. In an effort to streamline pesticide re-registration, nearly all of the organophosphate pesticides (e.g., Lorsban, Guthion, Malathion, Diazinon) have been assessed individually for aggregate, ecological, and worker risk. As of fall 2002, interim or final re-registraton eligibility decisions have been completed for most of these compounds. These decisions summarize the risk assessment conclusions and outline any measures necessary for the pesticide to continue to be registered in the United States, which may range from lowering application rates to complete cancellation of certain uses. As always, it is imperative to READ THE LABEL prior to using any pesticide to ensure legal application. The list of current decision documents is available at the EPA Office of Pesticide Program Web site at http://www.epa.gov/pesticides/reregistration/status.htm.
FQPA also required EPA to develop methods for assessing the "cumulative" risk of multiple pesticides that have a common mechanism of toxicity. A cumulative risk assessment is the process of evaluating the combined exposure (the amount of a pesticide to which an individual is exposed) and hazard (the health effects a pesticide could cause) from all substances that share a common mechanism of toxicity (i.e., that work the same way in the body). EPA released preliminary (December 2001) and revised (June 2002) cumulative risk assessments for the organophosphate (OP) group that excluded uses that were to be cancelled through risk mitigation actions. The revised risk assessment describes the potential risks of OPs by presenting a range of estimates of risk, stressing the inherent variability involved in this effort. EPA expects methods and knowledge to continue to evolve in this area and will update and/or revise the assessment as needed. The revised assessment and various summary documents are available at http://www.epa.gov/pesticides/cumulative/rra-op/.
The rigorous scientific and public processes followed by EPA during this tolerance reassessment and pesticide reregistration process continue to strengthen our confidence in the overall quality of our nation's food supply as one of the safest in the world. It represents the efforts of not only EPA staff but also significant contributions from a wide range of scientific experts, other stakeholders, and the public.
FQPA at the Regional Level
In recognition of the need for regional input on FQPA and reregistration decisions, EPA’s Strategic Agricultural Initiative was expanded in 2001. All ten EPA regional offices received staff and funding to help facilitate outreach, technical assistance, and grants for FQPA-related issues. Region 10 (AK, ID, OR, and WA) promotes Integrated Pest Management and FQPA-transition projects through partnerships with the land grant universities and agricultural organizations. A partial list of the projects sponsored in Region 10 is available at http://www.epa.prosser.wsu.edu.
In March 2001, American Farmland Trust (AFT) and EPA Region 10 announced grant awards for projects that reduce pesticide use or risk and increase the adoption of bio-intensive farming practices. Fourteen proposals, requesting a total of $601,005, were reviewed. The AFT-EPA cooperative agreement is based on AFT’s success with using the Outcome Funding framework, which requires on-farm cooperators and targeted-and-verified risk-reduction goals. The following proposals were funded with EPA Region 10 fiscal year 2001 Strategic Agricultural Initiative Funds.
Project Leader |
Location |
Project |
Risk Reduction Target |
Funding $ |
| Dr. Alex Stone | Willamette Valley, OR | increase use of IPM tactics on vegetable crops | 20% reduction in growers’ chlorpyrifos use | $49,689 |
| Phil VanBuskirk | Medford , OR & CA and WA | grower training on pesticide alternatives & use of weather data to forecast pests | 20% of pear growers will eliminate organophosphates | $23,500 |
| John Wells | Hood River, OR | BMPs in tree fruits to reduce OP use and improve water quality | 60% reduction in OP use by 30 growers; 100 growers will implement one or more water quality BMPs | $50,000 |
| Dr. Fran Pierce | Yakima Valley, WA | site-specific weather networks for integrated pest management | 10% reduction in pesticides on 25 collaborating farms by forecasting pest occurrence & intensity | $50,000 |
| Dr. Marcia Ostrom | Western WA carrot growers | alternative management strategies to reduce carrot rust fly including cover crops, row covers, intensive monitoring, and crop rotations | 25% of carrot growers will increase IPM tactics; 100% reduction in diazinon use by collaborating farms | $49,992 |
| Center for Ag Partnerships | WA tree fruit | Hispanic tree fruit grower risk pest management strategies | 12 Hispanic orchard owner/operators participate in development of education program and learn to use reduced risk practices | $49,900 |
Snapshots from some of the projects this summer show promise in meeting
their stated pesticide-risk reduction targets.
Hood River Grower-Shipper Association has begun its implementation of best management practices (BMPs) on tree fruits in northern Oregon with a goal of reducing reliance on OPs and improvement of water quality. Kristin Kerwin was hired as Project Coordinator. Kerwin has experience working with public participation and watershed policy in the Finger Lakes region of upstate New York. She is working with 300 pear, apple, and cherry growers across 11,500 acres in Hood River County, located in the Mid-Columbia Basin in northern Oregon. The goal of the Hood River Grower-Shipper Association is to help thirty growers achieve a sixty percent or greater reduction in the use of OP insecticides in areas adjacent to waterways and to assist at least 100 of the 300 growers in implementing one or more new pesticide BMP within “sensitive areas” to avoid contaminating water bodies. Initial activities have included drafting and sending an introductory letter and benchmark survey to the growers and conducting meetings with local fieldpersons. In addition, tours of local orchards and related project sites have been conducted in conjunction with Oregon State University field days. Kerwin developed a BMP presentation for pesticide license holders' Core Training Program and has compiled existing educational materials for the BMP project handbook. Tips for the growers include technical information on mating disruption, riparian buffers, irrigation water management, and sprayer maintenance and calibration, and information on the existing funding opportunities available to implement these practices.
The Site-Specific Weather Networks for Improved IPM project is a collaborative effort between Dr. Fran Pierce, director of the Washington State University (WSU) Center for Precision Agriculture and Drs. Martin Williams, Gary Grove, and Doug Walsh, WSU-Prosser research faculty in weed science, plant pathology, and entomology, respectively. The project is based in the mid- and lower-Yakima River Basin of eastern Washington and involves the development of low-cost, high-quality weather networks to help farmers predict outbreaks of insects, weeds, and plant diseases through refined pest modeling. This new generation of on-farm weather stations will allow growers to tailor data collection and products specific to their location and crop needs. As of mid-summer 2002, the group reported success in technology development, testing, and deployment of the Yakima Valley network backbone. One of the pilot stations operating at WSU-Prosser was used to predict early onset of powdery mildew, leading to precise timing and the use of preventative materials in fighting the fungal infection. Preliminary information about this project will soon be available through the Center for Precision Agricultural Systems' Web site at http://www.cpas.prosser.wsu.edu, then click on "AgWeatherNet."
Hispanic orchard owner/operators are the fastest-growing group of new producers, many of whom initially worked as employees and/or managers in other orchard operations before becoming growers. While many of these growers speak English, they often prefer educational information provided in Spanish. As small-acreage growers, they are typically not served by pest consultants and dealers. In addition, these growers should benefit directly from the use of reduced-risk practices since they both own the orchard and typically serve as the mixers, loaders, handlers, and applicators, and often involve family members in the orchard operations. The Center for Ag Partnerships originally proposed providing a targeted educational program to implement reduced-risk pest management by twelve Hispanic tree fruit growers. In the summer of 2002, thirty-six growers, more than three times as many as anticipated, began participating in the educational program conducted by Naná Simone. The growers are from the Chelan/Douglas and Tonasket areas. They differ in the size of their orchards, the cultivars grown, the age of their trees, the way in which fruit is marketed, and the management of their operations. Evening meetings are used to demonstrate scouting techniques, pest identification, pest models, and control options. Growers receive pesticide certification credits for participating in the meetings. For a more complete report on this project, with photos, see the Web page at http://www.agcenter.org/hisp/hisp.htm.
In early September 2002, a new call for proposals was issued by American Farmland Trust and EPA Region 10. The Request for Proposals may be found at http://www.aftresearch.org/grant/grant_info.php. The due date for proposals is November 1, 2002 with decisions on funding made by late December. Awards up to $50,000 per project will be available in February of 2003. Projects focusing on pest management with reduced-risk and bio-intensive techniques will continue to provide Pacific Northwest growers with solutions for production in a post-FQPA world.
Sandy Halstead is with EPA Region 10. Her office is located at WSU’s Irrigated Agriculture Research and Extension Center (IAREC) in Prosser, where she can be reached at (509) 786-9225 or halstead.sandra@epa.gov.
Return to Table of Contents for the October, 2002 issue
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Acrylamide AngstAnother Annoying Distraction About Food Safety |
Click here for PDF version of this document (recommended for printing). Should you not have Adobe Acrobat Reader (required to read PDF files), this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html |
Dr. Allan S. Felsot, Environmental Toxicologist, WSU
Media headlines this past summer proclaimed a new concern about a contaminant in food. Swedish researchers at the University of Stockholm reported that acrylamide was present in a variety of baked and fried goods (Tareke et al. 2002). Although the name sounds like something scary that a big, bad global corporation might be foisting on us, acrylamide is actually an all-natural molecule that forms in food during the cooking process. High-carbohydrate foods seem to have the highest levels.
The irony of this story is that while we have spent countless hundreds of millions of dollars since the passage of the Food Quality Protection Act addressing worries about pesticide residues in food, there has been a natural compound with some pretty nasty hazards right under our noses (not to mention in our stomachs if we’re eating a burger and fries while reading this). At least, that is the case if high-dose rat studies are valid indicators. Which just goes to show, we know a heck of a lot more about the highly regulated chemical products synthesized one-by-one in factories than we do about the plethora of natural chemicals produced during the chemistry of cooking.
While the Europeans, acting on the input of reports from the European Commission (ECSCF 2002), the Swedish National Foods Agency (SNFA 2002), and the World Health Organization (WHO 2002), seem to be particularly concerned about acrylamide and beg for more studies to figure out what it all means, it’s probably a good idea to take a breather and skeptically examine the hazard based on studies already in hand. After all, we humans have been exposed to this stuff ever since fire instigated our love affair with cooking.
Probably the best way to understand what the fuss is about is to use the risk assessment approach. Using this strategy, I will discuss acrylamide in the context of hazard identification, dose-response relationships, exposure characterization, and risk characterization. Finally, I will compare the purported risk with the reality of what we have been observing in the human population.
 Acrylamide
Acquaintance
Acrylamide
Acquaintance
The first stop on our journey is an overview of acrylamide. Acrylamide is a small molecular weight, highly water-soluble compound composed of carbon, hydrogen, oxygen, and nitrogen (Figure 1). But don’t let its simplicity fool you. It is quite reactive in air and is easily polymerized (i.e., single molecules of acrylamide can be coupled together to form a larger substance with new properties). The resulting polymer, known as polyacrylamide, has a variety of important uses:
- coagulant in sewage and wastewater treatment systems and for clarifying drinking water;
- binder for strengthening paper and paperboard products;
- additive to enhance oil recovery;
- soil stabilizer in the construction of dams, foundation, tunnels, and roadways;
- separation gel in analytical biochemistry work;
- grouts for construction and repairing, including sealing sewer pipe and tunnels;
- stabilizer, foam builder, binder, film former, antistatic agent, and hair fixative in various kinds of cosmetics.
Acrylamide Awareness
Pertinently, polyacrylamide always contains some unreacted acrylamide molecules, which are known as monomers. Thus, under the right circumstances humans can be exposed to acrylamide from a variety of sources. Indeed, concentrations of acrylamide in water are regulated by the EPA, and guidance levels have been set by the World Health Organization (WHO).
The current brouhaha about acrylamide has its origin in a serendipitous discovery associated with the use of about 1400 tons of grout to seal a railway tunnel in porous rock underlying crop and pasture fields in rural Sweden (Reynolds 2002). Acrylamide monomers leached from the sealant and contaminated ground and surface water. Fish floated dead, cows became paralyzed, and workers reported numbness. Investigations of the worker problems led to acrylamide as an immediate suspect because of the chemical’s well-known potential to cause neurotoxicity.
A Swedish professor at Stockholm University, Margaret Torqvist, was consulted to investigate the worker health issues (Reynolds 2002). Professor Torqvist had been investigating biomarkers for estimating worker exposure to acrylamide. Simply stated, biomarkers are very large cellular or blood plasma molecules (“macromolecules”) that can be directly measured to estimate exposure to contaminants. Typical measurements include changes in a biomarker’s biological activity in the presence of contaminants or an analysis for pieces of contaminants that bind to the biomarker. Pieces of the acrylamide molecule will bind to specific amino acids such as valine and cysteine on hemoglobin molecules; hemoglobin is the oxygen-carrying protein (i.e., the macromolecule) found in the red blood cells. When hemoglobin is digested, the amino acids with bound pieces of the acrylamide molecules (known as adducts) can be detected and quantified.
Torqvist’s research group noted unexposed subjects with comparatively high levels of acrylamide hemoglobin (Hb) adducts. Earlier, Torqvist’s colleague from Stockholm University, Emma Bergmark, reported that laboratory personnel working with polyacrylamide gels had detectable acrylamide Hb adducts (as predicted), but so did non-lab workers (Bergmark 1997). Cigarette smoke is known to contain acrylamide, but the control group participants were not smokers and other known sources of acrylamide exposure did not account for the magnitude of background levels of Hb adducts. Thus began the search for the source of the background acrylamide exposure. By process of elimination of known polyacrylamide sources in combination with the knowledge that heating vegetation (i.e., tobacco) caused elevated acrylamide Hb adducts, cooked food became the hypothesis to bet on.
Hazard Identification
Acrylamide is very rapidly absorbed through the intestinal tract and fairly evenly distributed throughout the body organs (Dearfield et al. 1988). However, with the exception of the testes, it does not accumulate in any particular tissue. Once exposure ceases, it is rapidly lost from the body. Acrylamide is metabolized by a mechanism known as conjugation; the compound is hooked onto glutathione, a molecule composed of three amino acids. Glutathione conjugates are rapidly excreted from the body. Enzymes known as microsomal oxidases transform some of the acrylamide to the molecule glycidamide (Figure 1), which can also be conjugated to glutathione (Sumner et al. 1997). Both acrylamide and glycidamide can bind to hemoglobin and presumably other proteins in the cells (Bergmark et al. 1993).
Despite acrylamide’s rapid metabolism and excretion following exposure, its high reactivity with proteins could be the reason it is hazardous to workers (Friedman et al. 1995). Because glycidamide also binds to DNA, it has been hypothesized to be the actual agent of toxicity (Segerback et al. 1995). Acrylamide has been well studied since the 1950s as a neurotoxin owing to its extensive use for polyacrylamide production (Dearfield et al. 1988). Its neurotoxicity was manifested symptomatically in workers through nerve tissue pathologies (axonopathies) and poor performance on neurological tests.
High doses of acrylamide can also cause adverse developmental and reproductive effects. For example, nerve degeneration and abnormal changes in intestinal enzymes have been observed in neonatal rodents (Dearfield et al. 1988). Abnormal sperm, reduced fertility, and spontaneous abortions have been elevated in treated rodents.
In the early 1980s, screening studies suggested that acrylamide could initiate tumors of the skin in orally or dermally exposed mice subsequently treated with an additional chemical known to be a very potent tumor promoter. Lung tumors were also noted in acrylamide-exposed mice not subsequently treated with a tumor-promoting agent (Bull et al. 1984). These observations were surprising in light of acrylamide’s failure to cause gene mutations in the usual bacteriological and mammalian cell culture studies of the time (Dearfield et al. 1988). However, when male mice were given non-lethal doses of acrylamide in drinking water and then allowed to mate with females, some fertilized eggs did not implant into the uterus, and some implanted embryos were aborted (Smith et al. 1986). This phenomenon is ascribed to dominant lethal mutations in the sperm cell chromosomes. Thus, acrylamide was hypothesized to cause chromosomal aberrations (known as clastogenicity) rather than DNA mutations (i.e., mutagenicity). On the other hand, the metabolite glycidamide can bind directly to DNA, but its mutagenic potential has been poorly studied (Segerback 1995; Tareke et al. 2000). Regardless of the specific mechanism of interacting with the genes, acrylamide has been classified as being genotoxic.
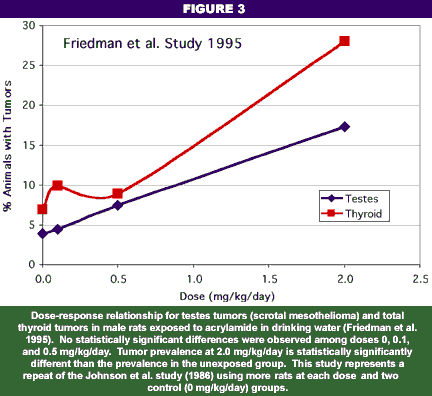
Rats given water containing acrylamide over a period of two years developed a number of different tumors. In one study (Johnson et al. 1986), tumors of the testes epithelium (a pathology known as scrotal mesothelioma) and the female mammary glands were elevated above control levels. In a later study (Friedman et al. 1995) involving a greater number of rats, only scrotal mesothelioma was definitively noted, but the significance for humans is obscure because this type of cancer is extremely rare. Nevertheless, on the basis of the Johnson et al. (1996) study and the classification of acrylamide as genotoxic (i.e., it causes mutations and/or chromosomal aberrations), the International Agency for Research on Carcinogenicity (IARC, an independent world authority for analysis of carcinogenic potential), EPA, and WHO consider acrylamide as a probable human carcinogen.
Current worries over acrylamide in food are directly related to the compound’s classification as a carcinogen. Indirectly, the uncertainty over acrylamide in cooked food is related to the hypothesis that genotoxins have no threshold for cancer causation. In other words, exposure to one molecule of a genotoxin can hypothetically kick off the biochemical process leading to cancer.
How Much
Is Too Much?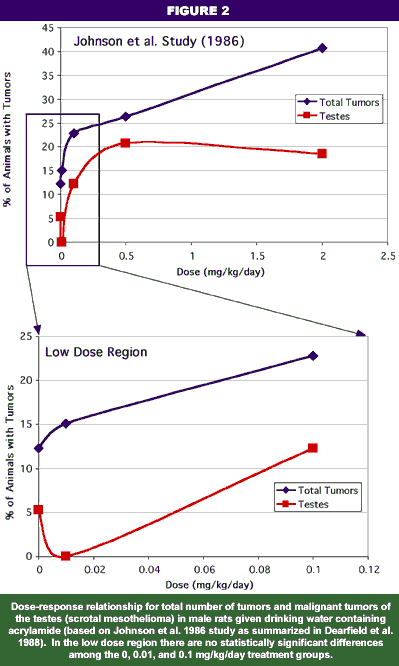
Hazard assessment usually starts with determination of the LD50, which is defined as the dose causing death to 50% of test subjects. The acute (i.e., single) oral LD50 for acrylamide in rats and mice is 107-270 mg/kg of body weight (WHO 1996). The acute dermal LD50 for rats was reported to be 400 mg/kg.
Definite thresholds and no-observable adverse effects levels (NOAELs) have been observed for both neurotoxic effects and developmental/reproductive toxicity at non-lethal subchronic doses (multiple doses for periods up to 90 days). The NOAEL for peripheral nerve lesions (the most sensitive endpoint for neurotoxicity) was noted in one study as 0.2 mg/kg/day (WHO 1996) and in another study as 0.5 mg/kg (Dearfield et al. 1988). Ten weeks of exposure to drinking water with acrylamide levels equivalent to whole body doses of 0.5 mg/kg/day (for pregnant rats) and 2.0 mg/kg/day (for fetal and neonatal rats) did not cause toxicity in developmental studies (Dearfield et al. 1988). For reproductive toxicity, 0.5 mg/kg/day given to male rats for 10 weeks caused no adverse effects on reproduction (Dearfield et al. 1988). Thus, neurotoxic, developmental, and reproductive effects are considered to have dosage thresholds that are exceeded when toxicity occurs. The relationship between dose and these effects is non-linear, but remarkably, the NOAEL of 0.5 mg/kg in adult rats is similar among different types of effects (Friedman et al. 1995).
In contrast to their treatment of non-carcinogenic effects, EPA, WHO, and IARC do not consider a threshold to exist in the relationship between acrylamide levels in drinking water and tumor formation. In other words, the agencies regulate genotoxins on the basis of the hypothesis that one molecule can cause an adverse effect on a gene, thereby initiating the process of tumor formation. Yet, close examination of the actual chronic (two-year) feeding studies for almost all chemicals shows that the lowest dose of the three doses usually tested does not cause any significant increase in tumors in exposed animals compared to the unexposed (control) animals (see Figures 2 and 3). Indeed, several reviews have shown a number of chemicals actually reduce the incidence of tumors in animals at low doses (Haseman and Johnson 1996; Crump et al. 1999). And so it is with acrylamide: the study upon which governmental agencies have relied to define hazard and its relationship with dose indicated no increase in tumors at the lowest dose tested (Johnson et al. 1986; EPA IRIS 1993). As a matter of record, a second chronic toxicity study (Friedman et al. 1995) showed that even the mid-dose did not cause any significant increase in tumors. The only significant and verifiable tumor increase was observed in the male testes at a dose of 2 mg/kg/day, and the conclusions argued for consideration of 0.5 mg/kg/day as a NOAEL for tumor formation.
 How
Much Is In Food?
How
Much Is In Food?
When Swedish researchers hypothesized that cooked food might be contributing to a “significant” background level of acrylamide exposure, they fried up some rat chow and served it to rats (Tareke 2000). They found the Hb biomarker levels in rats with this diet significantly elevated in comparison to the control rats. Chemical analysis of the fried chow revealed an average acrylamide concentration of 150 µg/kg (ppb), but none was detected in uncooked rat chow. Thus, this study started the search for acrylamide in other cooked foods.
As of October 2002, hundreds of food samples have been analyzed for acrylamide in several government, university, and private laboratories (Table 1). Examination of all the available data sets leads to the general conclusion that fried and baked foods of all kinds have acrylamide. The highest concentrations were observed in high carbohydrate foods, including potato and wheat products, but even high protein foods like meats have easily detectable levels of acrylamide. The good news is that acrylamide hasn’t been found in beer yet.
Newspapers have been serving up alarming stories of high acrylamide levels in French fries and potato chips. However, they have completely ignored the striking variability of acrylamide levels in the high carbohydrate foods. Acrylamide concentrations in multiple samples from any one food category have been ranging from non-detectable amounts (<5 to <30 µg/kg) to concentrations of low parts per million (mg/kg) (Table 1).
TABLE 1 |
||||
|
Ranges
of Acrylamide Concentrations (µg/kg) Found in Restaurant or
Purchased Foods As Reported in Various Studies |
||||
| Food Group |
CSPI |
SNFA |
ECSCF |
Tareke
et al. |
| French Fries | 250-423 |
300-1100 |
<50-3500 |
314-732 |
| Potato Chips |
881 |
330-2300 |
170-2287 |
1300-3897 |
| Corn Chips | 106-388 |
120-180 |
34-416 |
|
| Breakfast Cereals |
212-247 |
<30-1400 |
<30-1346 |
|
| Biscuits/Crackers | <30-650 |
<30-320 |
37-1731 |
|
| Soft Breads |
<30-160 |
<30-162 |
<5-53 |
|
| Bakery Products | <50-450 |
|||
| Hamburger & Pork |
23-45 |
|||
| Fish & Seafood | 30-39 |
|||
| Poultry or Game |
39-64 |
|||
| Drink Powders | <50-230 |
|||
| Beer |
<30 |
<5 |
||
|
All
reports were published in 2002; full citations are listed under
References. CSPI = Center for Science in the Public Interest; SNFA
= Swedish National Food Administration; ECSCF = European Commission
Scientific Committee on Food (note that these data combine the SNFA
data with those from Norway, Switzerland, U.K., and the U.S.); Tareke
et al. 2002 (Stockholm University). |
||||
The variability of acrylamide among the food groups can be viewed best by examining the distribution of residue concentrations in individual samples of specific high carbohydrate foods (Figure 4, below). With the exception of potato chips, the residue levels are overwhelmingly below 500 µg/kg with just a few unusually high detections. The residue levels in potato chips are more evenly distributed from the low to the high end. Thus, for most high carbohydrate foods, just examining the average residue level would tend to bias the perspective of how much acrylamide is generally in the food.
Studies are already proceeding full steam ahead to determine why cooking causes acrylamide formation, especially in high carbohydrate foods (Tareke et al. 2002). The first reports suggest that acrylamide does not form in boiled potatoes or meat (Table 2). When these same foods are fried (or, in the case of potatoes, even microwaved), detectable levels of acrylamide show up. Although the prevailing hypothesis focuses on high carbohydrate foods, fried spinach has surprisingly elevated levels of acrylamide also (Table 2).
TABLE 2 |
||
|
Acrylamide
Concentration (µg/kg) in Foods Prepared under Controlled Laboratory
Conditions (Tareke et al. 2002) |
||
| Food |
Preparation
1/ |
µg/kg |
| Potato, boiled or raw |
boiled
or raw |
<5 |
| Potato, grated, microwaved |
grated
& microwaved |
455-650 |
| Potato, grated & fried |
grated
& fried |
310-780 |
| Potato, boiled, mashed, fried |
boiled,
mashed & fried |
144-201 |
| Beetroot |
grated
& fried |
810-890 |
| Spinach |
grated
& fried |
112 |
| Beef |
boiled
or raw |
<5 |
| Beef |
minced
& fried |
15-22 |
| Cod |
boiled
or raw |
<5 |
| Cod |
microwaved |
<5 |
| Cod |
minced
& fried |
<5-11 |
| 1/ Boiled preparation was for 20 min; fried was for 2.5 min per side (beef, cod, and potatoes formed into patties) at 220°C without oil; microwaved was for 3 min on each patty side at 750 Watts. | ||
 Exposure
Assessment
Exposure
Assessment
Thousands of samples will probably be analyzed for acrylamide residues over the next year. Meanwhile, with residue distributions in hand, actual whole body exposure estimates are popping up like soufflés. One of the earliest exposure estimates stemmed from the biomarker work in Swedish lab workers (Bergmark 1997). Measurements of acrylamide Hb adduct biomarkers can be used in combination with information about the metabolism and excretion rate (i.e., pharmacokinetics) of acrylamide to calculate an exposure or intake dose. Among non-smoking subjects with no laboratory exposure to polyacrylamide, the average acrylamide exposure was estimated to be 0.8 µg/kg/day. Laboratory personnel who worked with polyacrylamide gels had estimated exposures of 1.4 µg/kg/day. Smokers who didn’t handle polyacrylamide were estimated to be taking up an average acrylamide dose of 3.1 µg/kg/day.
The use of biomarkers and pharmacokinetics are probably the best way to estimate human exposure. However, too few humans can be feasibly studied to garner population-wide estimates of exposure. For a broader view of exposure, therefore, food residues can be multiplied by the amount of food people typically eat. This type of exposure calculation is exactly what EPA uses for estimating dietary risk of pesticide exposure. Depending on who is doing the calculating, estimated average daily dietary exposures to adults have been ranging from 0.2 µg/kg to 0.8 µg/kg (ECSF 2002). The Swedish National Food Administration (SNFA 2002a) estimated an exposure from all sources as 100 µg/day; thus, for an adult of 70 kg (the standard “toxicological” weight), the daily whole body dose would be 1.4 µg/kg.
Betting on Acrylamide Exposure
The above exposure estimates give an average daily exposure. To conduct a risk assessment for an entire population, it is also desirable to know the distribution of exposures. In other words, some foods have low amounts of acrylamide while others have high amounts. This variation is true even for similar food products, like chips and fries. Also, some people eat a lot of certain foods one day and very little the next. Furthermore, adults eat different amounts of snack foods than do kids. Thus, to gain insight into a population-wide exposure to acrylamide, toxicologists can run a probabilistic estimation of dietary intake.
A probabilistic assessment of dietary exposure would randomly select one food sample and an associated residue from the available database of residues (see Figure 4 for a distribution of acrylamide residues by food category). This residue would then be multiplied one at a time by the amount of that food eaten by one individual recorded in a food consumption database (for further explanation of the use of probabilistic analysis see Felsot 2002). The probabilistic analysis employs a computer modeling technique called Monte Carlo analysis. This little calculation game is like playing cards, where the toxicologist randomly selects a card from the acrylamide residue deck and pairs it with a randomly selected card from the food consumption database deck.
Probabilistic dietary exposure was recently assessed for people living in Netherlands (Klaveren and Boon 2002). The residue data were taken from the developing acrylamide residue databases in the United Kingdom, Sweden, and Norway. The result of the analysis is actually a distribution of exposure values. For long-term exposure (i.e., daily lifetime exposure), the average of the distribution is relevant; it was estimated to be 0.8 µg/kg/day for adults and 1.83 µg/kg/day for young children. High-end exposures are estimated from the upper five percent of exposures; the 95th percentile of exposure was estimated at 3.1 µg/kg/day and 6.4 µg/kg/day for adults and children, respectively. In other words, 95% of the adult population may be exposed to acrylamide at a dose of 3.1 µg/kg/day or less. Pertinently, these comparatively high exposure levels only represent a short-term (one to a few days) exposure, not a daily lifetime exposure.
Risk Characterization: MOEs vs. Models
To characterize the risk or probability of adverse non-carcinogenic effects from acrylamide exposure, agencies typically examine the ratio of the NOAEL to the exposure level. The magnitude of this ratio, known as the Margin of Exposure (MOE), should be within agency risk guidelines to conclude a “reasonable certainty of no harm.” For example, if the NOAEL for the most sensitive neurotoxic effect caused by acrylamide is 0.2 mg/kg/day (i.e., 200 µg/kg/day), and the short-term exposure at the 95th percentile is 3.1 µg/kg/day, then the MOE is 65. In other words, 65 times less acrylamide is consumed at the high end of exposure than is associated with a complete absence of the most subtle neurotoxic effect in rodents. Based on the upper end estimate of average daily intake of 0.8 µg/kg/day, the MOE is 250.
By the way, both rodents and humans are believed to be susceptible to neurotoxic effects from acrylamide exposure, and furthermore, the rate of uptake from the intestine and subsequent distribution and metabolism seems similar (Dearfield et al. 1988). Thus, rodents may be reasonable sentinels for humans with regard to non-carcinogenic effects. Indeed, based on current knowledge of dietary exposure levels, regulatory agencies worldwide are not concerned that acrylamide residues in food will cause adverse neurological, developmental, or reproductive effects.
Regulatory concern over carcinogenic effects of acrylamide is a different story. The policy basis for the MOE approach is the hypothesis that non-carcinogenic effects have clear thresholds below which health effects are nil. When it comes to genotoxicity and carcinogenicity potential, however, the regulatory agencies switch their game and invoke the hypothesis that a single exposure (i.e., one molecule) can lead to cancer. In this case, officials do not rely on an empirically derived NOAEL (i.e., a dose directly observed in experimentation). Rather, they pull out the old linear dose-response computer models and extrapolate the curve down to low doses that were never tested. The result of this mathematical exercise is the derivation of a slope for the linearized dose-response curve that represents the tumor rate, or the number of tumors per milligram of dose per kilogram of body weight per day (tumors/mg/kg/day). Based on this extrapolation of high dose data to low dose virtual exposures, EPA had estimated a slope factor of 4.5/mg/kg/day (EPA IRIS 1993).
Using the slope factor of 4.5 and an assumed exposure of 1 µg/kg/day (i.e., 0.001 mg/kg/day), EPA has calculated a lifetime risk of 4.5 excess cancers per 1000 population (EPA IRIS 1991). Using different models but the same exposure factor, other calculations of risk for cancer offer probabilities of 0.7 per 1000 and 10 per 1000 (ECSCF 2002). The results of these calculations should not be interpreted to mean that 10 people will actually get cancer from short-term exposures to acrylamide. Rather, these risks represent the probability that X number of people in a population of 1000 will develop cancer if exposed to acrylamide over a lifetime. Such a probability should be weighed against the current estimated lifetime risk for cancer of 330 per 1000 (SNFA 2002b).
If the prevailing regulatory hypothesis had been that a threshold actually does exist for tumor development in rodents, then a NOAEL approach could have been used for estimating the lifetime cancer risk from exposure to acrylamide. In this case, the MOE could have been calculated from the Friedman et al. 1995 study for the statistically significant tumors (NOAEL = 0.5 mg/kg/day) and a dietary intake of 0.001 mg/kg/day. The resulting MOE would be 500. This value could be interpreted as saying that the current dietary intake levels of acrylamide are 500-fold lower than the dose associated with no significant tumor development in rats exposed daily for two years.
Is the No-Threshold Hypothesis Relevant?
The wisdom of the no-threshold hypothesis for carcinogenicity has been increasingly criticized over the last decade (e.g., Ames and Gold 1993; Ames et al. 1993; Cohen and Elwein 1992; Clayson 1998; Goodman 2001). Critics urge EPA to examine the mechanisms of toxicity, not just count the tumors, before making a blanket decision on whether a compound is a probable carcinogen. Nevertheless, WHO and the European Union have treated acrylamide as a very dangerous, high-potency carcinogen by accepting lock, stock, and barrel the hypothesis of no threshold for putative genotoxins. Yet, a closer examination of acrylamide’s hazards with regard to chronic toxicity suggests a compound that might actually be working through hormonal actions, rather than directly on the gene (EC 2001). This hypothesis stems from observations that reproductive tissues (e.g., sperm, testes, mammary glands) seem to be particularly vulnerable to high-dose effects of acrylamide.
Acrylamide itself does not readily bind to DNA; however, one of its metabolites (glycidamide) does when injected into the body cavity of rats at high doses (50 mg/kg) (Segerback et al. 1995). Glycidamide adduct levels associated with background acrylamide exposures have been too low to accurately measure (Bergmark 1997). Glycidamide Hb adducts, however, have been measured in highly exposed acrylamide industry workers in China, especially when neurological pathologies were noted (Bergmark et al. 1993).
Bear in mind several operational principles regarding biomarker adducts. First, DNA adducts are generally repaired as long as the cell is healthy (Ames et al. 1993). Secondly, compounds forming Hb adducts can bind to a lot of different proteins, including the proteins surrounding the DNA in the chromosomes (Friedman et al. 1995). The fact that acrylamide exposure at high doses causes chromosome aberrations (as opposed to gene mutations) suggests that deformations in the proteins put strains on the DNA strands, resulting in breakage (Friedman et al. 1995). If this hypothesis is correct, then acrylamide tumorigenic effects, which are only seen at unreasonably high doses in rats and mice, occur in such a way that repeated interactions or hits are required. In other words, acrylamide’s biochemical interactions fit a profile that favors invoking the threshold paradigm. Finally, glycidamide resulting from high dose administration of acrylamide may bind with DNA of different rodent tissues, but failure to find accumulation of adducts in the testes tissue suggests that the tumors noted in this site (Johnson et al. 1986; Friedman et al. 1995) may not be due to a genotoxic effect (Segerback 1995). In other words, a threshold may exist for tumor formation.
Another reason for being skeptical of the no-threshold hypothesis for acrylamide comes from a careful examination of the chronic toxicity study (Johnson et al. 1986) relied upon for the risk assessment. Graphical examination of the results of the most significant tumors in the two-year drinking-water study shows a few percentage points’ difference between tumor prevalence in the control group and the group given up to 0.1 mg/kg/day (Figure 2). None of the observed differences are statistically significant (Dearfield et al. 1988). Buried in the Johnson et al. (1986) report are other extenuating circumstances that have raised questions about its utility for risk assessment (Friedman et al. 1995). For example, at the highest doses, significant numbers of rats died and many of the remainder showed evidence of nerve pathologies, raising the issue that cellular toxicity rather than genotoxicity was operational. Also, some of the subject rats were reported to have a viral infection, again raising concerns over stressors that could enhance the toxicity of acrylamide.
More importantly for purposes of risk characterization, the experiments in the Johnson et al. study were repeated by Friedman et al. (1995), and the conclusions were somewhat different. The Friedman et al. study strongly argued that a clear NOAEL of 0.5 mg/kg/day existed for the only significant tumor observed, scrotal mesothelioma (Figure 3). Thyroid tumors, which had been used by the NSFA to estimate cancer risk were not significant in the updated study. Furthermore, all of the genotoxicity studies that have measured clastogenicity of acrylamide or dominant lethal effects also show clear threshold effects (i.e., the lowest tested doses generally show no adverse effect; e.g., Smith et al. 1986). In short, the regulatory agencies have been basing their risk assessment partly on a chronic toxicity study that has been superceded with a better designed study. Regulators continue to ignore the repeated result of no effect associated with the lowest dose tested.
Reality Checks
Risk communication about the discovery of acrylamide in cooked foods has ranged from commendable to awful. The United Kingdom (UK) Food Standards Agency has communicated well using a question-and-answer format to inform consumers about what we know and don’t know, and to communicate a rational approach for eating a balanced diet (UK FSA 2002). On the other hand, WHO officials expressed alarming themes with quoted sound bites such as, “After reviewing all the available data, we have concluded that the new findings constitute a serious problem” (WHO/FAO 2002). “We know we get a lot of cancers from food, some of it might come, or it is very likely that it does come, from acrylamide" (ABC NewsOnline 2002). “…[G]iven that we know acrylamides are cancer-causing in animals and probably in humans, it is intolerable that they are in foods at the levels found and we have to find a remedy” (Kaufman 2002).
FOLKS, IT’S TIME FOR A REALITY CHECK.
In addition to the points made in the above discussions about the relevancy of the no-threshold hypothesis, other observations give pause to pushing the panic button. Occupational epidemiological studies with workers, the most highly exposed human population, are useful as sentinels for excess cancer risk in the population. At least three different studies of acrylamide factory workers have been published that concluded no excess mortality from any disease, including cancer (Marsh et al. 1999). If acrylamide was as potent a carcinogen as the regulators (and WHO officials) believe, then a look at over 8500 workers exposed between 1925-1994 should tell us something, especially with regard to cancers of the testes and thyroid gland. Yet, no associations between exposure and cancer mortality at any organ site were found (Marsh et al. 1999).
Considering that cooked foods have been a staple of our diets since the advent of fire, our exposure to acrylamide is both ancient and unavoidable. Concern has been greatest over the inordinately high levels of acrylamide in cooked, high-carbohydrate foods, but that type of exposure has been with us since the cultivation of the first grains. By clinging to the hypothesis that there is no threshold for acrylamide’s tumorigenic effects, we have painted ourselves into a proverbial risk corner. If there is truly no threshold, then regulatory officials need to explain why many types of cancers in addition to lung cancer are either falling or have stabilized in incidence rate at a time when we are supposedly eating a lot more of these high-carbohydrate, cooked foods (Wingo et al. 1998; Wingo et al. 1999).
Is it worth worrying about naturally occurring substances that test out as rodent carcinogens? Consider the conclusions of the National Research Council report, “Carcinogens and Anticarcinogens in the Human Diet” (NRC 1996), which pointed out that natural products and synthetic chemicals in the diet are “present at levels below which any significant adverse biologic effect is likely, and so low that they are unlikely to pose an appreciable cancer risk.”
One theme propounded by everyone
is the need to eat a well-balanced diet with plenty of fruits and vegetables.
If this advice is faithfully followed, then why would anyone want to back
off potatoes, no matter how they are cooked? This vegetable, whether fried,
baked, or boiled, can provide up to 40% of the recommended daily dose
of cancer-fighting ascorbic acid (Vitamin C) (NRC 1996; OECD 2002).
Here’s my risk communication message: next time that you drive up
to your neighborhood fast food joint, have it your way. Just remember
to order a mixed salad with those super-size fries!
Dr. Allan S. Felsot is with the Food and Environmental Quality Laboratory at Washington State University's Tri-Cities campus. A frequent contributor to AENews, he can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
References
ABC News Online. 2002. Serving up cancer. Australian Broadcasting Corporation June 28, 2002. http://www.abc.net.au/news/indepth/featureitems/acrylamide.htm.
Ames, B. N. and L. S. Gold. 1993. Environmental pollution and cancer: some misconceptions. Phantom Risk, K. R. Foster, D. E. Bernstein, and P. W. Huber, ed., MIT Press, Cambridge, MA: pp. 153-181.
Ames, B. N. et al. 1993. DNA lesions, inducible DNA repair, and cell division: Three key factors in mutagenesis and carcinogenesis. Environmental Health Perspectives Supplements 101(Suppl. 5):35-44.
Bergmark, E., C. J. Calleman, F. He, and L. G. Costa. 1993. Determination of hemoglobin adducts in humans occupationally exposed to acrylamide. Toxicology Applied Pharmacology 120:45-54.
Bergmark, E. 1997. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 10:78-84.
Bull, R. J., M. Robinson, R. D. Laurie, G. D. Stoner, E. Greisiger, J. R. Meier, and J. Stober. 1984. Carcinogenic effects of acrylamide in Sencar and A/J mice. Cancer Research 44:107-111.
Center for Science in the Public Interest (CSPI). 2002. New tests confirm acrylamide in American Foods. http://www.cspinet.org/new/200206251.html.
Clayson, D. B. 1998. Is the use of linear low-dose extrapolation still justified for carcinogens? Regulatory Toxicology and Pharmacology 28:69-70.
Cohen, S. M. and L. B. Ellwein. 1992. Risk assessment based on high-dose animal exposure experiments. Chem. Res. Toxicol. 5:742-748.
Crump, K. S., D. Krewski, and C. Van Landingham. 1999. Estimates of the proportion of chemicals that were carcinogenic or anticarcinogenic in bioassays conducted by the national toxicology program. Environ. Health Perspectives 107(1):83-88.
Dearfield, K. L., C. O. Abernathy, M. S. Ottley, J. H. Brantner, and P. F. Hayes. 1988. Arcylamide: its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutation Research 195:45-77.
EPA Integrated Risk Information System. 1993. Acrylamide. http://www.epa.gov/iris/subst/0286.htm.
European Commission (EC) Scientific Committee for Toxicity, Ecotoxicity and the Environment. 2001. Opinion on the results of the Risk Assessment of: ACRYLAMIDE (Human Health and the Environment). http://europa.eu.int/comm/food/fs/sc/sct/out88_en.html.
European Commission Scientific Committee on Food (ECSCF). 2002. Opinion of the scientific committee on food on new findings regarding the presence of acrylamide in food. SCF/CS/CNTM/CONT/4 Final, 3 July 2002 http://europa.eu.int/comm/food/fs/sc/scf/index_en.html.
Felsot, A. S. 2002. Necessity is the mother of invention. EPA unveils preliminary CRA on OPs. Agrichemical & Environmental News (January) 189, 15 pp. http://www.aenews.wsu.edu/Jan02AENews/Jan02AENews.htm#CRAonOPs.
Friedman, M. A., L. H. Dulak, and M. A. Stedham. 1995. A lifetime oncogenicity study in rats with acrylamide. Fundamental and Applied Toxicology 27:95-105.
Goodman, J. I. 2001. Operational reversibility is a key aspect of carcinogenesis. Toxicological Sciences 64(2):147-148.
Haseman, J. K. and F. M. Johnson. 1996. Analysis of National Toxicology Program rodent bioassay data for anticarcinogenic effects. Mutation Research 350:131-141.
Kaufman, M. 2002. Panel calls acrylamide in food a 'serious' risk. ProHealth Network.com. http://www.prohealthnetwork.com/library/showarticle.cfm/ID/418/.
Johnson, K., S. Gorzinski, K. Bodner, R. Campbell, C. Wolf, M. Friedman, and R. Mast. 1986. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol. Appl. Pharmacol. 85:154-168.
Marsh, G. M., L. J. Lucas, A. O. Youk, and L. C. Schall. 1999. Mortality patterns among workers exposed to acrylamide. Occupational & Environmental Medicine 56(3):181-190.
National Research Council (NRC). 1996. Carcinogens and anticarcinogens in the human diet. National Academy Press, Washington, D. C. p. 5.
OECD (Organization for Economic Cooperation and Development). 2002. Consensus document on compositional considerations for new varieties of potatoes: key food and feed nutrients, anti-nutrients and toxicants. Series on the Safety of Novel Foods, No. 4; ENV/JM/MONO(2002)5, Environmental Directorate, OECD, Paris, France. http://www.oecd.org/ehs/.
Segerback, D., C. J. Calleman, J. L. Schroeder, L. G. Costa, and E. M. Faustman. 1995. Formation of N-7-(2-carbamoyl-2hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis 16:1161-1165.
Smith, M. K., H. Zenick, R. J. Preston, E. L. George, and R. E. Long. 1986. Dominant lethal effects of subchronic acrylamide administration in the male Long-Evans rat. Mutation Research 173:273-277.
Sumner, S. C. J., L. Selvaraj, S. K. Nauhaus, and T. R. Fennell. 1997. Urinary metabolites from F344 rats and B6C3F1 mice coadministered acrylamide and acrylonitrile for 1 or 5 days. Chem. Res. Toxicol. 10:1152-1160.
Swedish National Food Administration (SNFA). 2002a. Analytical methodology and survey results for acrylamide in foods. http://www.konsumentverket.se/html-sidor/livsmedelsverket/engakrylanalysresultat.htm.
Swedish National Food Administration (SNFA). 2002b. Acrylamide-cancer studies and comparisons of risk. http://www.konsumentverket.se/html-sidor/livsmedelsverket/engakrylcancerstudier.htm.
Tareke, E., P. Rydberg, P. Karlsson, S. Eriksson, and M. Tornqvist. 2002. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 50:4998-5006.
U.K. Food Standards Agency (UK FSA). 2002. FSA acrylamide study: your questions answered. http://www.foodstandards.gov.uk/multimedia/webpage/acrylamide_study_faq/.
van Klaveren, J. and P. Boon. 2002. Dietary exposure assessment of acrylamide in the Netherlands. Draft report for FAO/WHO consultation on the health implications of acrylamide in food, WHO, Geneva, 25-27, June 2002.
Wingo, P. A., L. A. G. Ries, H. M. Rosenberg, D. S. Miller, and B. K. Edwards. 1998. Cancer incidence and mortality, 1973-1995. Cancer 82(6):1197-1207.
Wingo, P. A., L. A. G. Ries, G. A. Giovino, D. S. Miller, H. M. Rosenberg, D. R. Shopland, M. J. Thun, and B. K. Edwards. 1999. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J. National Cancer Institute 91(8):675-690.
World Health Organization (WHO). 1996. Acrylamide. pp. 541-547 in Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. WHO, Geneva, Switzerland. http://www.who.int/water_sanitation_health/GDWQ/Chemicals/Acrylamfull.htm.
WHO/FAO. 2002. Additional research on acrylamide in food essential, scientists declare. Joint WHO/FAO Press Release/51 June 27, 2002. http://www.who.int/inf/en/pr-2002-51.html.
Return to Table of Contents for the October, 2002 issue
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Announcements & Upcoming ConferencesEPA Releases Methyl Bromide BrochureThe US Environmental Protection Agency has released a brochure entitled "Phaseout of Methyl Bromide." The brochure includes the deadline and process for filing for critical exemption, facts about the chemical's use, and other information users need to know now and in the future. It is not too late to apply for critical use exemption. The brochure is in the process of being printed and is currently available in Portable Document Format (PDF) by clicking here. (If you cannot read PDF files, you may download Adobe Acrobat Reader free of charge at http://www.adobe.com/prodindex/acrobat/readstep.html.) PIC Becomes WSPRSWashington State University's Pesticide Information Center (PIC) has become Washington State Pest Management Resource Service (WSPRS). The change in name reflects a change in emphasis from an exclusive focus on traditional pesticides to a broader perspective encompassing the entire spectrum of pest management, including integrated pest management (IPM), biological and cultural control, organic pest management, and other areas. WSPRS will continue to serve pesticide information needs by housing a complete collection of pesticide labels registered in Washington and Oregon, maintaining the Pesticide Information Center On-Line (PICOL) label and tolerance databases (http://picol.cahe.wsu.edu/LabelTolerance), and providing the Pesticide Notification Network (http://www.pnn.wsu.edu). WSPRS is also home to Agrichemical and Environmental News. The staff remains the same and the functions will not diminish; the new name is simply a more accurate representation of the work that is actually being done now and the directions the office plans to take in the future. Watch for more information about WSPRS in an upcoming AENews feature article. See
the new Web page!
|



















