


To see this report in Portable Document
Format (PDF), please click on the button. ![]() Should you not have Adobe
Acrobat Reader (required to read PDF files), this free program
is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Should you not have Adobe
Acrobat Reader (required to read PDF files), this free program
is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Open Forum: In an attempt to promote free and open discussion of issues, The Agrichemical and Environmental News encourages letters and articles with differing views. To discuss submission of an article, please contact Dr. Allan Felsot at 509-372-7365 or afelsot@tricity.wsu.edu or Dr. Catherine Daniels at 509-372-7495 or cdaniels@tricity.wsu.edu. The newsletter is available in a hardcopy version for a $15 yearly subscription fee. Please contact newsletter editor Sally O'Neal Coates at 509-372-7378 or scoates@tricity.wsu.edu for details.
This page has been accessed times since February 23, 2000.
Representatives of the Environmental Protection Agency (EPA) Office of Pesticide Programs held a technical briefing in Pasco, Washington, on February 10, 2000, on the review and reregistration status for phosmet. Approximately eighty-four growers, commodity representatives, consultants, and other stakeholders assembled at the Doubletree Inn to listen and respond to a six-member EPA panel representing the Special Review and Reregistration Division, the Biological and Economic Analysis Division, and the Health Effects Division.
The meeting came at the close of phosmet's Phase 4 of the six-phase Pilot Review Process for organophosphates (OPs) as delineated by EPA's Tolerance Reassessment Advisory Committee (TRAC). (See "TRAC Pilot OP Review Process," below.) Phase 4 is a ninety-day period during which EPA revises its preliminary risk assessment and holds public meetings such as this one to inform stakeholders of the content of the revised risk assessment. Phase 4 sets the stage for Phase 5, sixty days during which EPA actively solicits and considers risk management ideas. The revised risk assessment was to be available on the EPA website by the end of the third week of February, beginning the Phase 5 comment period.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phosmet is an insecticide used on a variety of fruit and vegetable crops, nut trees, cotton, ornamentals, and forest sites. Its primary formulation is the commercial preparation known as Imidan®. Phosmet is also used by home gardeners and as a livestock and dog treatment. EPA estimates an average of one million pounds of phosmet are applied each year, with apples and peaches representing 42 and 11 percent of use, respectively.
Phosmet, like other organophosphates, is a cholinesterase inhibitor. Simply stated, overexposure to such compounds can lead to excitation of the nervous system causing nausea, dizziness, confusion, and even respiratory paralysis and death. No grave human health incidents have been documented resulting from phosmet exposure.

EPA presented a written summary statement covering dietary risk, residential exposure risk, worker risk, drinking water risk, and ecological risk. As dietary, drinking water, and aggregate dietary (food + water) risks were determined to be of no concern, these segments of the presentation were brief. Residential exposure presented some hazards, particularly for toddlers coming in contact with treated pet dogs. Other residential hazards could include homeowners' use of phosmet in home gardens and in the treatment of pet dogs. Ecological risks of concern included risk to birds and mammals under chronic (repeated) application conditions. Some aquatic risks have been identified, and phosmet is highly toxic to honeybees. Due to lengthy discussion on worker risks, ecological risks were not discussed or presented in detail at this briefing, but are available in the revised risk assessment documentation.
The initial segments of the morning's presentation were useful and appropriately brief. Special Review and Reregistration Division Director Lois Rossi opened the meeting with an introduction and overview of EPA's OP review process, and continued to ably moderate the balance of the day's presentation and discussion. Diane Isbell from the same office gave a regulatory history of phosmet and William Gross, with EPA's Biological and Economic Analysis Division, presented use and usage profiles for the pesticide. Christina Swartz, with the Health Effects Division, gave the good news about food and water residues.
Where things began to break down was in the area of worker exposure and risk, presented by Health Effects Division's Jeffrey Dawson as part of his occupational and residential risk assessment overview. While EPA was interested in telling the group about the multifarious scenarios they considered in revising the phosmet documents (twenty-two distinct age groups considered for each exposure scenario; five categories of exposure, each with subgroups; inclusion of very low-level exposures such as irrigation maintenance), the attendees wanted to know the hardcore specifics pertinent to their industry:
"Why did you use nothing but pear data for apples?"
"Why, if 75 percent of blueberries are washed post-harvest, and phosmet is a surface residue, are blueberry statistics drawn as if all fruit is unwashed?"
Of course, a major EPA objective in technical briefings such as this one, and in the sixty-day Phase 5 comment period that follows, is to expose data gaps and proceed to refine data. This objective was reiterated throughout the approximately three hours of audience testimony.
While phosmet's widespread use in the apple industry brought EPA to the nation's apple capital for this briefing, attendees came from throughout the country to air their concerns. The sometimes-impassioned testimony from the floor followed two basic themes: the importance of maintaining phosmet's current status, including its short re-entry intervals (REIs), and specific errors or gaps in EPA's data that need refinement.
A Gowan Company representative (Gowan is the registrant for Imidan) praised EPA's thoroughness, but expressed a problem with the REI calculations, asking that real-world data be incorporated both in terms of the interval itself and the actual exposure a worker might realize. Gowan also asked for careful reconsideration of the 10x interspecies "safety" factor, postulating that human and rat reaction might be the same. (Where compounds are tested on animals, the dose without effect is divided by 10, assuming greater human sensitivity in the absence of data to the contrary.)
 A California almond
grower took issue with two points. Nuts are mechanically harvested,
yet the EPA assessment to date uses the same data for nuts as
for (manually harvested) apples when calculating post-harvest
worker exposure. (A Michigan cherry representative expressed a
similar concern.) Also, EPA used the same active-ingredient-per-acre
(ai/A) numbers for different nuts, while actual practices vary
between nut types.
A California almond
grower took issue with two points. Nuts are mechanically harvested,
yet the EPA assessment to date uses the same data for nuts as
for (manually harvested) apples when calculating post-harvest
worker exposure. (A Michigan cherry representative expressed a
similar concern.) Also, EPA used the same active-ingredient-per-acre
(ai/A) numbers for different nuts, while actual practices vary
between nut types.
Several growers and commodity representatives explained that many Imidan applications are border treatments, directly affecting only perimeter trees.
An apple harvest photo shown as part of EPA's presentation was criticized. The photo depicted a picker on a ladder surrounded by foliage, misrepresenting a growing preponderance of dwarf trees, harvest of which involves less foliar contact.
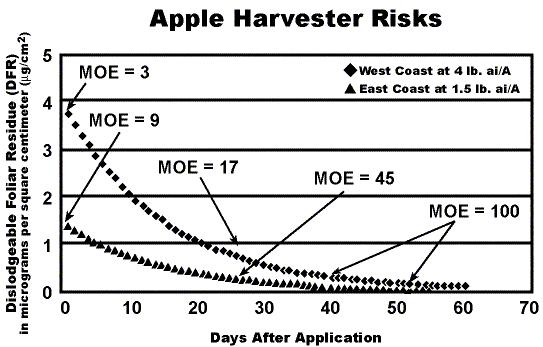
Another slide in the presentation drew a great deal of attention. Titled "Apple Harvester Risks," the graphic showed two margin of exposure (MOE) curves for pickers: an "east coast" scenario at 1.5 lb active ingredient per acre (ai/A) and a "west coast" scenario at 4 lb ai/A. (A reconstructed facsimile of the slide is presented as Figure 1.) MOE is a concept unique to worker exposure assessments. It is derived by dividing the NOAEL (No Observed Adverse Effects Level, expressed in milligrams per kilogram of body weight per day) by the dose, also expressed in mg/kg/day. The higher the resulting number, or MOE, the lower the risk. Where EPA got into trouble was in stating, "the ideal MOE is 100." An apple grower pointed out that if a worker re-entered the orchard at seven days after application-a common practice and permissible under the current label-he or she would be entering at a MOE of about 12. Simply put, "where are the dead bodies?" At this point, EPA representative Dawson became rather defensive, offering such helpful rejoinders as, "It's very complicated," and (my favorite), "Our toxicologist isn't here" In the end, under the assured steering of Director Rossi, it was re-explained that MOEs are predicated on NOAEL endpoints-an endpoint of no adverse effect, not of death. Accordingly, risk management can aim for numbers short of the "ideal" (in this case, short of 100) and still be well within safe exposure limits.
|
|
 |
|
|
A Washington apple grower pointed out some specific discrepancies between the phosmet pear data used by EPA for apples and actual apple practices. The pear data gave an application rate of 4 lb ai/A, where actual west coast apple rates are 3 to 3.5 lb ai/A. Management practices applied to apples and not to pears, such as overhead cooling by sprinkler irrigation, can also affect residues.
Referring again to the controversial MOE curve slide (Figure 1), a New York grower wondered why if, according to the curve, residues of phosmet remain after thirty-five days, they need to spray every seven days for plum curculio? He also pointed out that rainfall ("a rather frequent occurrence in our part of the world") is known to "rapidly diminish" Imidan effectiveness, and suggested this real-world phenomenon be considered with respect to residues and worker risk.
A San Joaquin Valley (California) grower explained that Imidan is a crucial part of his Integrated Pest Management (IPM) program. Last year, he treated only thirty-three of his 390 acres-less than ten percent-with only one application. Without the ability to use phosmet as an emergency tool, and to use it with suitably short REIs-as short as twenty-four hours in some cases-he would have to resort to preventative (more frequent) sprays, perhaps with harder chemistries. The need to maintain the 24-hour REI on certain crops was echoed by many speakers.
 Another San Joaquin
Valley grower expressed his frustration with trying to respond
to international markets where zero tolerance of pests is the
norm and pesticide residue concern is secondary. He saw Imidan
as a bridge between the U.S. demand for softer controls and the
international demand for zero pests.
Another San Joaquin
Valley grower expressed his frustration with trying to respond
to international markets where zero tolerance of pests is the
norm and pesticide residue concern is secondary. He saw Imidan
as a bridge between the U.S. demand for softer controls and the
international demand for zero pests.
Several speakers, including a Wapato, Washington, apple grower, questioned EPA's assumption of eight hours' exposure in a worker's eight-hour day. In real life, a picker will be up and down the ladder throughout his or her shift, performing a range of tasks, and at most might contact foliage five or six hours in the day. Another grower pointed out that scouts and other workers contact foliage even less, perhaps only an hour or two a day. EPA explained that their monitoring process took a variety of typical work activities into consideration over the workday.
While most testimony focused on worker risk, a Michigan blueberry grower and a California stone fruit grower pointed out a dietary consideration. The baby food market-one of our nation's strictest in terms of allowable residues-is tolerant regarding Imidan.
After the testimony, after the explanations, and after competing with the shouts, huzzahs, and something resembling singing from the adjacent Kiwanis Club meeting, EPA agreed to consider several points in refining data, among them: mechanical vs. manual harvest, border sprays vs. overall block treatments, and better characterization of re-entry activities.
The main disconnect between EPA's presentation and the concerns of the attendees was that of scope. In an attempt to address the admittedly nebulous and broad mandates of the Food Quality Protection Act, EPA evaluates a vast universe of possible exposure scenarios. If this universe were represented by, say, a basketball, the concerns of the growers and others testifying at the February briefing could be represented by a pea or a ping-pong ball. The nation at large includes those who think all produce should be organic and that global warming should be a number one priority; these individuals are part of the public that EPA serves when they dissect every conceivable exposure scenario and divide it by twenty-two age groups, generating a set of numbers for each. The agricultural community, on the other hand, is not terribly impressed with reams of data that all say, "it's safe, it's safe, it's safe," followed by an offhand mention that this "little matter" of worker exposure is "not safe." Especially when the data used to say "not safe" is, well, is comparing apples to oranges. Or, in this case, pears.
The worker risk data need to be refined. Some of the ecological data do, too. No one on either side of the podium was denying that at the Pasco conference. Now is the time to contribute to that refinement.
By the time this article reaches readers, the revised risk assessment for phosmet should be available at www.epa.gov. Comments are being accepted at opp-docket@epa.gov or by mail at
U.S. EPA OP Pesticide Docket (7502C) 401 M St. SW Washington, DC 20460Phosmet (Imidan) questions may be directed to Diane Isbell at isbell.diane@epa.gov or (703) 308-8154. Gowan Company is coordinating information on re-entry activities, utilizing an EPA-developed matrix addressing various practices, their duration, and their timing relationship to phosmet. To participate in pro-viding this information, contact Cindy Baker, Gowan's Director of Governmental Affairs, at (520) 819-1554 or cbaker@gowanco.com. The sixty-day comment period began with the posting of the revised risk assessment, so the time to provide input is now.
Sally O'Neal Coates is Editor of Agrichemical and Environmental News. She can be reached at (509) 372-7378 or scoates@tricity.wsu.edu.
Dateline: October 30, 1938. The popular U.S. weekly radio show, Mercury Theater of the Air, is interrupted to make an astounding and frightening announcement. The broadcaster, in a stressed but deadpan voice, declares the countryside under attack by an invading force of Martians. Thousands of people react in panic: jamming switchboards, demanding information, preparing to leave the city (just in case).
Fast Forward: Summer 1999 to Winter 2000. Newsweek runs a headline questioning the safety of "Frankenstein Foods." Demonstrators clad in lab coats and gas masks rip corn plants from research plots in France and the United Kingdom to save the world from "genetic pollution." A major snack food producer announces it will no longer accept genetically engineered corn to make its nutritious, oily, and salt-laden treats, apparently out of grave concern about our health.
 Alas, on the precipice
of a new millennium, the western world is a healthier, wealthier,
and safer place in which people can worry themselves to death
with perceived threats around every corner.
Alas, on the precipice
of a new millennium, the western world is a healthier, wealthier,
and safer place in which people can worry themselves to death
with perceived threats around every corner.
Surely, on that Halloween night sixty-two years ago, Orson Welles could not anticipate the paranoia triggered by his now-famous mock news broadcast. Did he assume his radio fans would instantly get the joke and understand his thinly veiled allusions to H.G. Wells' War of the Worlds?
Perhaps the Monsantos and Novartises of the world, producers of genetically engineered corn, soybean, cotton, and potato seed, anticipated modern man would instantly understand the benefits of gene technology. Did they assume that consumers worldwide would be knowledgeable enough to weed out misinformation from truth?
Based on the media headlines and sound bites today, perhaps Welles and the multinational companies assumed too much. Over 30% of the surveyed public in Europe responded "true" to the statement that non-genetically engineered foods do not contain genes (11). Alien genes have become the new alien beings, and "we got trouble in River City."
 You thought synthetic
chemicals were bad? Meet the big, bad GENE. Reluctance on the
part of our European trading partners to accept genetically engineered
food could lead to serious consequences for our food production
system. One of the present worries is the insertion of a gene
from the naturally occurring bacterium Bacillus thuringiensis
(Bt) into corn, cotton, and potato to produce a transgenic crop
that would confer resistance to insect feeding.
You thought synthetic
chemicals were bad? Meet the big, bad GENE. Reluctance on the
part of our European trading partners to accept genetically engineered
food could lead to serious consequences for our food production
system. One of the present worries is the insertion of a gene
from the naturally occurring bacterium Bacillus thuringiensis
(Bt) into corn, cotton, and potato to produce a transgenic crop
that would confer resistance to insect feeding.
The inserted bacterial gene directs the synthesis of a protein highly toxic to some major pest insects. When certain insects feed on the crop foliage, they become sick, stop feeding, and eventually die. One way of viewing the new genetic trait is simple host-plant resistance, a long-desired tool for integrated pest management (IPM) usually acquired through many years of conventional plant breeding. The technology seems to hold great potential for substantially reducing the use of sprayed pesticides and consequent contamination of nontarget areas. Haven't we taught our children that protein is good for them and pesticide residues are bad?
Nevertheless, fear of genetic modification technology has engulfed Europe and threatens to spread to the United States. Already, grain handlers and food processors in the United States are reacting to the closing of European markets by demanding segregation of genetically modified and unmodified seeds. Why is there such concern over a natural substance, a gene and its protein? Why are Europeans more emotional about genetic modification than they are about the use of synthetic pesticides?
Part of the reason for a reluctance to accept genetic modification of crops may lie in a general unfamiliarity with basic cell biology and the ecological distribution of native Bt. Ignorance of biology shows in the previously mentioned European poll about genes. Bt's natural ubiquity may be misunderstood because it can be formulated as a commercial spray that is registered with the Environmental Protection Agency (EPA) as a pesticide. In Europe, this lack of understanding has been exacerbated by recent food "scandals" including mad cow disease, tainted Coke®, and dioxins in Belgian chickens.
Fearmongering is raised to an art form by Greenpeace, one of the most vocal environmental advocacy groups on this issue (http://www.greenpeace.org). Arguments raised by Greenpeace and others against genetic engineering include:
The first five concerns on the list are testable hypotheses; the last two are purely social politics, not open to scientific inquiry. Greenpeace vehemently rants against all types of genetic engineering, but for now, just trying to understand fear of Bt engineered products is a big enough task. Because governments worldwide have expressed concerns similar to the seven outlined by Greenpeace, each point merits dissection with respect to Bt transgenics. First, let's take a look at the biology of native Bt and review how we leapt from native genes to transgenes.
Bt was first isolated in 1901 from a diseased
silkworm moth in Japan. In 1911, E. Berliner isolated a similar
microbe from a diseased flour moth in Germany; he gave Bt its
current scientific name (16). The association
of Bt with insect pathogenicity suggested its application as an
insecticide to control the European corn borer (Ostrinia nubialis)
in Europe during the late 1920s. Inquiries into the factors responsible
for Bt's pathogenicity did not begin until the 1950s and culminated
in the late 1980s with an understanding of the molecular basis
of its toxic mechanism (5).
When nutrients are plentiful and pH and temperature are favorable (as in an insect body), Bt grows rapidly and reproduces asexually by simple cell division (a.k.a. vegetative growth). As nutrients in its immediate environment become limited, Bt cells produce a spore that only germinates when conditions become favorable again.
At the time of sporulation, Bt also produces a crystalline proteinaceous inclusion called the parasporal body. When certain insect species incidentally ingest the sporulated Bt cells with their parasporal body, the alkaline midgut (i.e., insect digestive tract) solubilizes the crystalline parasporal body releasing a protein toxin known as the delta-endotoxin (5). Then a midgut enzyme unwittingly cleaves the endotoxin into the actual toxin that eventually kills the insect.
The toxic protein fragment binds to specific molecular receptors on the midgut cells, causing the membranes to lose their integrity and the gut tissue to swell up (5). The insect stops feeding and eventually starves to death. A dying insect is probably the most favorable environment for Bt growth and reproduction. As the insect body completely decays due to bacterial septicemia, the spores and proteins disperse into the environment waiting to be ingested by other unsuspecting insects.
Bt spores and proteins are found ubiquitously in soils, plant foliage, and stored grains, but growth in those environments has not been proven. Indeed, epizootics (i.e., disease outbreaks) of Bt among insects are rare if they occur at all. Bt spores may be fairly stable in soil after an initial extensive degradation and/or predation by other soil microorganisms (13). On plant foliage, the spores and crystal proteins are subject to degradation if exposed to direct sunlight. Thus, the amount of Bt available to susceptible insects may be too limited to cause a natural outbreak of disease.
The first commercial Bt products were simply fermentation cultures of isolates having similar host specificity and potency as the original isolates. A product called Sporeine was available in 1938 in France for control of flour moths (16). The first commercial product in the United States, Thuricide, appeared in 1957.
Bt products deployed in agriculture and forestry prior to the 1970s produced inconsistent results. Bt seemed to be pathogenic only to very specific species in the order Lepidoptera (moths and butterflies). Furthermore, it was toxic only to young larvae. In 1970, a new isolate of Bt was discovered that was up to 200 times more active against targeted pests. This new isolate, which represented a new subspecies, was called kurstaki and was given the appellation HD-1. Bt kurstaki HD-1 became the gold standard against which to compare the potency of all future Bt isolates.
 And the isolates kept
on coming. Initial discoveries showed that different Bt strains
were pathogenic to different species of the order Lepidoptera.
Yet Bt was not a general insect pathogen. In the 1970s a strain
toxic to primitive flies of the order Diptera (mosquitoes and
blackflies) was isolated and named subspecies israelensis. By
1980, a commercial product was being sold for control of mosquito
and blackfly larvae; aquatic invertebrates and fish were unaffected
by this new strain (11).
And the isolates kept
on coming. Initial discoveries showed that different Bt strains
were pathogenic to different species of the order Lepidoptera.
Yet Bt was not a general insect pathogen. In the 1970s a strain
toxic to primitive flies of the order Diptera (mosquitoes and
blackflies) was isolated and named subspecies israelensis. By
1980, a commercial product was being sold for control of mosquito
and blackfly larvae; aquatic invertebrates and fish were unaffected
by this new strain (11).
In 1982, a new Bt strain named subspecies tenebrionsis was isolated from a dead pupa of the yellow mealworm beetle, Tenebrio molitor (order Coleoptera) (6). Bt tenebrionsis was particularly pathogenic to beetles in the family Chrysomelidae (a.k.a. leaf beetles, which includes the Colorado potato beetle, Leptinotarsa decemlineata).
Today approximately 280 unique Bt strains have been isolated from insects, soils, foliage, and grain dust (http://epunix.biols.susx.ac.uk/Home/Neil_Crickmore/Bt/toxins.html). New strains are differentiated by the characteristics of their crystalline protein, their gene sequence, and their spectrum of insecticidal activity. All the strains have been organized into major groupings depending on their spectrum of insecticidal activity (Table 1).
|
|
||
|
|
|
|
| kurstaki HD-1 | Lepidoptera, Diptera | CryIA(a), CryIA(b), CryIA(c), CryIIA, CryIIB |
| thuringiensis HD-2Ý | Lepidoptera | CryIA, CryIB |
| aizawai | Lepidoptera | CryIA(a), CryIA(b), CryIC, CryID |
| entomocidus | Lepidoptera | CryIA(a), CryIB, CryIC |
| tenebrionis | Coleoptera | CryIIIA |
| israelensis | Diptera | CryIVA, CryIVb, CryIVC, CryIVD |
| *All endotoxins are named by the suffix Cry which stands for crystalline protein and a number and letter system to designate affinities in toxicity and genetic specificity. CryI are specific toxins for Lepidoptera, CryII are specific for Lepidoptera and secondarily some Diptera, CryIII are specific for Coleoptera, and CryIV are specific for Diptera. | ||
| ÝBt thuringiensis also produces an exotoxin (extracellularly secreted) that can be toxic to non-insect organisms. This strain is not used commercially in Bt products. | ||
|
|
||
Bt's tremendous diversity is due in part to the genetic information that controls the formation of the parasporal body. This information resides outside the chromosome on pieces of DNA called plasmids. The plasmids of any one Bt cell can be exchanged with or transferred to other Bt cells in a type of mating process called conjugation. Indeed, in nature one Bt strain may have created new strains by recombination of the DNA between conjugating cells (7).
Different subspecies of Bt have a penchant for attacking very specific groups of insects. Thus far, no other invertebrate or vertebrate animal seems susceptible to the toxic protein. This target specificity results from the specific protein structure, the nature of the insect midgut, and the receptors in the gut membranes.
To be effective, the crystalline protein must first be solubilized. Only the insect midgut has a high enough pH to effect dissolution. Second, protein-cleaving enzymes known as proteases must be able to snip off only part of the protein to produce the true toxin. Third, the clipped protein must recognize and bind to specific receptors in the membranes of the midgut cells.
The genetic diversity of the numerous subspecies of Bt has resulted in the synthesis of proteins toxic to certain insects and innocuous to others. The susceptible insects themselves carry genes that code for the synthesis of the receptors specific to the various protein toxins. This close relationship between Bt and its insect hosts is a classic example of the complementary evolution of a parasite and its host.
The specificity of different Bt strains
for their insect hosts is an advantage in pest control because
nontarget organisms are unharmed. On the other hand, this specificity
coupled with Bt's lack of environmental persistence detracts from
its usefulness. Bt is easy to grow in fermentation cultures, but
it must be sprayed on foliage surfaces, making it accessible only
to insects feeding on the leaf surfaces it contacts. Bt sprays
are ineffective against insects feeding on the bottom or uncontacted
surface of the leaf as well as insects that have burrowed inside
the plant tissue. Bt's susceptibility to degradation by sunlight
necessitates frequent spraying of crops with high pest infestations.
One way to improve the field effectiveness of Bt has been through optimization of sprayer technology. Production of optimally sized droplets coupled with changes in formulation have enabled Bt to be used quite successfully to control larvae of forest attacking insects, including the spruce budworm and the gypsy moth (16). By the late 1980s millions of forest acres had been sprayed, avoiding the use of the more controversial and broader spectrum conventional pesticides.
Another way to improve the utility of Bt has been through manipulation of its genes. In the early 1980s, the plasmid containing the toxin gene was transferred successfully into Escherichia coli bacteria, making it possible to sequence the gene (i.e., determine the DNA code) and develop probes that could be used to screen isolates for DNA sequences associated with the toxin (16). E. coli was the first transgenic Bt organism, but its use was purely for research to understand the gene structure and how gene expression was regulated.
The discovery of the endotoxin genes and their diverse specificity enabled efforts to make Bt a broad-spectrum insecticide by combining genes from different subspecies. Gene manipulation without genetic engineering (a.k.a. recombinant DNA technology) such as that used with the aforementioned E. coli experiments have been used successfully to bring several different toxic protein genes into a single Bt strain (4). Ecogen Inc. has developed products toxic to both Coleoptera and Lepidoptera by using conjugation between two Bt strains. Products of higher potency have been similarly produced for controlling lepidopteran forest and vegetable insects.
Electroporation is another method for introducing the plasmid containing Bt toxin into different Bt strains (4). When bacterial cells are subjected to an electrical field, pores open up in the membranes, allowing the DNA-containing plasmids to enter the cell. The endotoxin CryIIIA gene (Table 1), which is active against certain beetles, was transferred to the Bt israelensis strain that is toxic to mosquitoes. The resulting "improved" strain not only had activity against Diptera (mosquitoes) and Coleoptera as predicted, it also exhibited activity against Lepidoptera. Similarly, when a plasmid with the Lepidopteran-active CryIA(b) gene was transferred to Bt tenebrionis, the transformed strain was also toxic to mosquitoes. The unexpected activity of the manipulated Bt strains suggested that the endotoxin proteins could interact synergistically to expand toxicity to insect species not affected by either toxin alone.
Recombinant DNA technology has been used to improve the stability of Bt sprays. Mycogen Corp. engineered the toxic protein gene into the common soil bacterium, Pseudomonas fluorescens (4). The cultured bacteria express the protein, but do not produce a spore. The cells are killed and then formulated into a spray containing the encapsulated Bt protein. Because the organisms are dead, the regulations concerning release of live transgenic organisms are not applicable to the product. The encapsulated protein is significantly more resistant to light degradation than the native Bt spray formulation. The technique, known as CellCap, has resulted in four different EPA registrations (http://www.epa.gov/oppbppd1/biopesticides/ai/nonviable_microbials.htm).
Stability, ease of delivery, and greater pest control diversity, all highly desirable properties, have extended the usefulness of Bt. With the discovery that desirable genes could be cloned into the crown gall bacterium, Agrobacterium tumefaciens, a plant itself could become the delivery system for the Bt toxin.
 Agrobacterium cells
grow in intimate association with plant cells and can transfer
their DNA to the plant, which will incorporate the bacterial genes
into its own chromosomes. The Bt gene cloned into Agrobacterium
was actually a pared-down version of the native gene. Only the
code necessary for making the protein toxic remained, along with
pieces of DNA called promoters that allowed the recipient cells
to read the code.
Agrobacterium cells
grow in intimate association with plant cells and can transfer
their DNA to the plant, which will incorporate the bacterial genes
into its own chromosomes. The Bt gene cloned into Agrobacterium
was actually a pared-down version of the native gene. Only the
code necessary for making the protein toxic remained, along with
pieces of DNA called promoters that allowed the recipient cells
to read the code.
After the plant cells are transformed, the whole plant is reconstituted, but now all of its tissues express the toxin. By the late 1980s, tobacco, tomato, potato, and cotton had been transformed to express the Bt toxin using the Agrobacterium gene transfer system (2).
A unique way of expressing the protein in a plant involved cloning the gene into an endophytic bacterial species Clavibacter xyli. Endophytic bacteria invade the vascular system of plants (4). Corn seed is inoculated with the engineered bacteria, which replicate inside the plant and express the toxic protein. This novel method of inserting the toxin without transforming the plant genome was developed in the product InCide by the Crop Genetics International Corporation. However, the product is not currently registered.
Transgenic crops currently on the market are being produced from plant cell cultures that are literally shot full of the modified genes coding for one or more specific protein toxins. The endotoxin gene and its promoters are coated onto microscopic metal spheres that are literally fired at high velocities into the plant cell culture. The spheres enter the plant and, through a process of fusion, the DNA on the spheres is incorporated into the plant's genome.
Not every cell, however, will become transformed during this process. Thus, marker genes are used to help select the successfully transformed cells as well as to track the toxin gene as the plant grows and eventually reproduces. Modern Bt transgenic crops incorporate one or two types of markers that are spliced to the Bt toxin gene.
Some transgenic lines contain the PAT gene that codes for the enzyme phoshinothricin acetyl transferase. The enzyme occurs naturally in certain bacteria, and it confers tolerance to the herbicide phosphinothricin, which is a rare, naturally occurring amino acid produced by two soil microbes known as actinomycetes (12). After cells are shot with the genes, the herbicide can be applied to the culture; cells that have not been successfully transformed die. Cells with the PAT gene live and can be reconstituted into whole plants.
A gene that codes for antibiotic resistance is also spliced into the endotoxin gene but it is not functional in the cell. One of the antibiotic resistance markers, neomycin phosphotransferase (npt), is not expressed, but its well-known DNA sequence can be probed after the plants are grown to determine if they are transgenic or native (i.e., isogenic). The antibiotic resistance genes occur naturally and are widely distributed in environmental bacteria, so the likelihood of transgenic markers increasing antibiotic resistant microbes is nil (3).
Both Bt spray formulations and transgenic
crops must be registered by the EPA. Both are subjected to the
same toxicological testing. Indeed, one of the reasons that transgenic
Bt crops have been commercialized so rapidly is that the long
history of Bt use has demonstrated no toxicity to nontarget organisms
(9).
Bear in mind that human exposure to Bt proteins is ancient considering
that studies show it is widely distributed in soil, foliage, and
stored grain (8, 10, 14).
But transgenic crops receive even greater scrutiny than Bt sprays. The proteinaceous endotoxins and marker enzymes are tested for allergenicity. Various plant parts are ground up and fed to different invertebrate and vertebrate test organisms. The potential for gene transfer to other crop species, especially those that are closely related, is assessed. Most importantly, a plan for managing the development of resistance must be reviewed and approved. From an entomologist's viewpoint, the development of resistance is the greatest potential problem with using Bt transgenic crops. Nevertheless, under the current guidelines, EPA has already registered seven transgenic corn, potato, and cotton hybrids containing different endotoxin gene systems.
In summary, Bt and certain insects have coevolved a tight relationship between protein toxins and molecular receptors. Research efforts have focused on discovering Bt's diversity and broadening its spectrum of insecticidal activity. The disadvantages of conventional Bt sprays, including lack of persistence, narrow spectrum of activity, and lack of accessibility to internal plant pests, have been overcome through manipulation of the gene. Gene manipulation makes a lot of people uncomfortable-to the point of calling for a ban on transgenic technology. In Part II, I'll examine the safety record of natural Bt and transgenic crops, specifically addressing the concerns of Greenpeace and the European Community.
Dr. Allan S. Felsot is an Environmental Toxicologist with WSU and a frequent contributor to AENews. He can be reached at afelsot@tricity.wsu.edu or (509) 372-7365.
Return to Table of Contents for the March 2000 issue
What is your sector of agriculture doing in response to the Food Quality Protection Act (FQPA)? When I look around at the various sectors I see some focusing on a regulatory solution to force the Environmental Protection Agency (EPA) to work under certain constraints (bill H.R. 1592, "Regulatory Fairness and Openness Act of 1999"). Others are busily gathering actual data on pounds of pesticide applied, acres of crop treated, and so forth, to counteract EPA's admittedly conservative default assumptions. Other groups are using this opportunity to organize their industries to take a critical look at what drives pesticide use and which areas to target for research on alternatives.
 Why this last approach?
In 1998, the newly formed USDA Office of Pest Management Policy
(OPMP), looked down the road and predicted no matter how much
"sound science" was collected and reviewed, or how much
pesticide use and usage data had been evaluated, or how much "real
world" information had been obtained to mitigate risks, EPA
might still cancel uses of certain pesticides. If that happened,
agriculture needed to instantly be in a position to negotiate
with EPA for a phase-out period of targeted pesticides or face
collapse of many minor crop industries. Without information on
key pests, possible alternatives to the cancelled uses, influences
on existing IPM programs, and good estimates on the time necessary
to effect a transition, agriculture would be in a very weak bargaining
position. Taking this as an operational starting point, OPMP staff
started beating the drums for industry to develop some sort of
blueprint for this transitional phase. Initially, these conceptual
documents were referred to as "transition strategies."
The newly minted term is "pest management strategic plans."
Why this last approach?
In 1998, the newly formed USDA Office of Pest Management Policy
(OPMP), looked down the road and predicted no matter how much
"sound science" was collected and reviewed, or how much
pesticide use and usage data had been evaluated, or how much "real
world" information had been obtained to mitigate risks, EPA
might still cancel uses of certain pesticides. If that happened,
agriculture needed to instantly be in a position to negotiate
with EPA for a phase-out period of targeted pesticides or face
collapse of many minor crop industries. Without information on
key pests, possible alternatives to the cancelled uses, influences
on existing IPM programs, and good estimates on the time necessary
to effect a transition, agriculture would be in a very weak bargaining
position. Taking this as an operational starting point, OPMP staff
started beating the drums for industry to develop some sort of
blueprint for this transitional phase. Initially, these conceptual
documents were referred to as "transition strategies."
The newly minted term is "pest management strategic plans."
A pest management strategic plan (PMSP)
is a document that:
Necessary transition steps might include accelerated registration of a pesticide currently in the registration pipeline; investigation into the biology of a pest to identify more sensitive points in its life cycle for better control; and/or determination of the economic cost to the industry if pesticide A were substituted for pesticide B, including what economic returns industry would need to survive that transition. To oversimplify, you could say a PMSP tells why you are doing what you are doing now, then gives a step-by-step blueprint for how you can do it differently and still stay in business.

Sponsored workshops to develop PMSPs (known, albeit controversially, throughout 1999 as "transition strategies") have been held for several industries: California almonds, Southeastern apples, California peaches, Western pome fruits, Southeastern strawberries, and Michigan carrots. Depending upon the size and complexity of the industry, workshops have been scheduled for up to two days. A manageable workgroup should not exceed twenty people and should be made up of a mix of individuals including researchers and growers. A tremendous amount can be accomplished when concerned stakeholders meet for a day or two. From personal experience I can say that something as simple as feeding them lunch goes a long way toward keeping the meeting focused and on time.
At the western region pome fruit workshop held in Yakima, Washington, on November 19 and 20, 1999, participants included apple and pear growers, packers, pest control advisors, farm advisors, technical experts (researchers and specialists in entomology, horticulture, and IPM), and representatives from industry organizations. Representatives from EPA were also invited to attend. Wilfred Burr of OPMP and I moderated the workshop. The goals this workgroup chose were:
All industries, regardless of size or level of organization, are encouraged to go through the process of developing a pest management strategic plan. Many groups contemplating compiling a formal PMSP document will find they already have some of the elements on hand.
Those industries that have participated in the completion of crop profiles (see AENews Issue No. 154, February 1999) will find the work is already half completed. (See state-by-state crop profiles at http://ipmwww.ncsu.edu/opmppiap/proindex.htm, or PDF versions of Washington State profiles at http://www.tricity.wsu.edu/~cdaniels/wapiap.html.)
Many industries, such as the pome fruits, have already identified research areas through ongoing efforts of industry committees, and it is a simple matter to incorporate such information into a PMSP document. Industries that have not identified research areas will find that going through the process of writing the PMSP will assist them, or their land grant university researchers, in prioritizing their concerns. Many states still have faculty members who participated in the (now retooled) Pesticide Impact Assessment Program; these individuals would be able to facilitate a PMSP workshop.
The document resulting from the Western pome fruit workshop is still in draft form with an anticipated publication date of early spring 2000. Workshop proceedings from some of the other workgroups have already been published and are available from OPMP by contacting Wilfred Burr at (202) 720-8647 or wburr@ars.usda.gov.
Per Vice President Gore's instructions in his famous April 8, 1998, memo (see AENews Issue No. 145, May 1998), EPA is in close contact with USDA (specifically, OPMP) during the risk mitigation process (when each active ingredient goes through reregistration). OPMP staff are in the best position to effectively use the transition strategies to both buy time for minor crop producers and input EPA on which "pipeline" pesticides to fast track.
 If PMSPs are so useful,
why isn't every industry rushing to produce one? For some, it
has been a problem of semantics. The moniker "transition
strategy" that has been used over the past year has caused
some groups to reject the idea out of hand. A "transition
strategy," by name and definition, is based on the premise
that organophosphates (OPs) are being phased out. "Transition,"
in this context, precisely and pointedly referred to transitioning
from OPs to other control mechanisms. Starting with the premise
that OPs will be lost--despite the truth of it--hasn't sat well
with some groups.
If PMSPs are so useful,
why isn't every industry rushing to produce one? For some, it
has been a problem of semantics. The moniker "transition
strategy" that has been used over the past year has caused
some groups to reject the idea out of hand. A "transition
strategy," by name and definition, is based on the premise
that organophosphates (OPs) are being phased out. "Transition,"
in this context, precisely and pointedly referred to transitioning
from OPs to other control mechanisms. Starting with the premise
that OPs will be lost--despite the truth of it--hasn't sat well
with some groups.
Calling the documents "pest management strategic plans" is more than a matter of shifting semantics. The concept of transition strategies has always embraced much more than finding substitutes for OPs; the new name reflects that. Accordingly, the new documents will include all pesticide classes, not just organophosphates.
Rather than debate the nuances of vocabulary, perhaps we should look at practicality. If EPA is paying attention to anybody in agriculture, it would be USDA's OPMP. Call them transition strategies or call them PMSPs, these documents stand a good chance of being mutually accepted vehicles for imparting critical industry information.
Most agricultural producers would agree that a strategy derived by their industry would fit them a whole lot better than one derived by EPA. Wouldn't you?
Dr. Catherine Daniels is the Pesticide
Coordinator at Washington State University and has been the Pesticide
Impact Assessment Program (PIAP) liaison for Washington State.
She can be reached at (509) 372-7495 or cdaniels@tricity.wsu.edu.
Return to Table of Contents for the March 2000 issue
New concerns about pesticide health risks and children in the late 1980s were the foundation for the 1996 Food Quality Protection Act. Those concerns also spawned new efforts among public health scientists. We saw the need for a better understanding of exposure if we were to produce more accurate estimates of risk. Equally important, we needed to identify special populations at high risk.
Risk is often defined as the probability of harm. Groups at increased risk are normally those who either have high exposures or enhanced susceptibility to a particular disease agent. In the case of pesticides, for example, mixers, loaders, and applicators are considered "high risk" because of the relatively high exposure that can result from direct contact with commercial products and spray. Children are considered "high risk" because of possible increased susceptibility and the ongoing development of their organ systems.
 So what about children
of pesticide handlers and others who work with agricultural chemicals?
Aren't their risks potentially high both from the point of view
of exposure and of susceptibility? Our studies here at the University
of Washington School of Public Health and Community Medicine for
the past eight years have tried to answer these questions. We
decided that children in farming communities should be defined
as a special population for research, and that we needed to find
out if their exposures and risks were different from those of
other children. Furthermore, we knew that children in farming
communities were probably exposed to more than one pesticide,
and that pesticides that work by a common mechanism of action
may produce an additive or cumulative risk. In the end we decided
to focus our efforts on younger children (1-6 years old), and
we examined their exposure to the organophosphorus (OP) insecticides.
Nearly all OP pesticides have a similar mode of action: they inhibit
the nervous system enzyme acetylcholinesterase.
So what about children
of pesticide handlers and others who work with agricultural chemicals?
Aren't their risks potentially high both from the point of view
of exposure and of susceptibility? Our studies here at the University
of Washington School of Public Health and Community Medicine for
the past eight years have tried to answer these questions. We
decided that children in farming communities should be defined
as a special population for research, and that we needed to find
out if their exposures and risks were different from those of
other children. Furthermore, we knew that children in farming
communities were probably exposed to more than one pesticide,
and that pesticides that work by a common mechanism of action
may produce an additive or cumulative risk. In the end we decided
to focus our efforts on younger children (1-6 years old), and
we examined their exposure to the organophosphorus (OP) insecticides.
Nearly all OP pesticides have a similar mode of action: they inhibit
the nervous system enzyme acetylcholinesterase.
A major challenge for population-based exposure assessment studies is defining the study population. Sometimes this is done geographically or on the basis of existing databases such as census data. Ideally, a probabilistic sample can be drawn from a well-defined population so that results can be generalized to the larger population.
Defining "agricultural communities,"
however, turned out to be complicated. Such communities are widely
dispersed and do not always conform to census or political boundaries.
Once the community is defined, traditional methods of access to families may not be feasible. Among agricultural workers, multiple families may live in residences designed for a single family, and telephone-based sampling methods may miss a significant fraction of the population. In our state's agricultural regions the primary language of many workers is Spanish, so bilingual capabilities are essential.
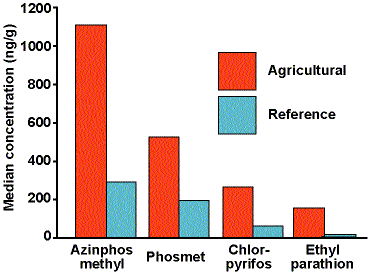
The area selected for our studies centered around Wenatchee, Washington. The region consists of an urban zone along the Columbia River, with orchards extending into the surrounding mountain canyons as well as upriver, and newer residential development interspersed with farmland. This entire region was considered the "agricultural community" for our studies. Orchard management in the area includes periodic application of several OP pesticides, including azinphos-methyl, chlorpyrifos, diazinon, phosmet, and malathion.
In our recent studies we attempted probability-based sampling using census tract data, but this approach required a randomized door-to-door contact, as much of the population did not have telephone service. We also found that families were wary of strangers approaching their doors, and were often unreceptive to our request for participation. This method was ultimately abandoned as impractical. Study participants were recruited through community organizations, including social service agencies, clinics, and producer-operated cooperatives. This approach allowed us to quickly identify families with young children.
Our studies in 1992 and 1995 divided households into two groups based on proximity to farmland and parental occupation. "Agricultural" families were defined as households that included at least one adult working in farming. Adult workers were further classified as pesticide applicators and farm workers in the 1995 study. None of the pesticide applicators in these studies conducted this activity full-time. A smaller "reference" family population was also recruited. These families had no household members working in farming, and lived more than one-quarter of a mile (about 400 meters) from farmland. Children up to six years of age were recruited from these families. Often more than one child per family would participate in the study.
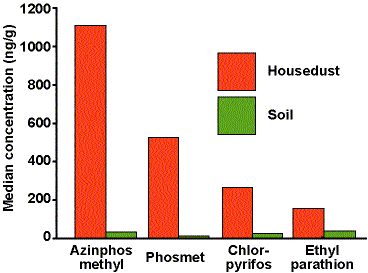
When we began this work in 1991 there were no laboratories prepared to conduct multiple OP residue analysis in media other than food. Even acquiring appropriate standards was problematic. Our lab had to develop new analytical methods to meet our needs for environmental measurements. Our 1992 and 1995 studies focused on four OP pesticides used in Washington state orchards: azinphos-methyl, phosmet, chlorpyrifos, and ethyl parathion. We included soil and housedust sampling.
 Thirty OP pesticides
were registered for use in Washington State in 1998. Studies expanded
to include diazinon, dichlorvos, malathion, methyl parathion,
methidathion, mevinphos, ethoprop, phorate, dimethoate, and terbufos;
sample media were expanded to include twenty-four-hour indoor
air, indoor and outdoor surface wipes, and drinking water. Duplicate
one-day diet samples were analyzed by Dr. Carol Weisskopf at the
Food and Environmental Quality Laboratory at Washington State
University. Nonetheless, more than half of the OP pesticides registered
in Washington State still fell outside these analytical capabilities.
Thirty OP pesticides
were registered for use in Washington State in 1998. Studies expanded
to include diazinon, dichlorvos, malathion, methyl parathion,
methidathion, mevinphos, ethoprop, phorate, dimethoate, and terbufos;
sample media were expanded to include twenty-four-hour indoor
air, indoor and outdoor surface wipes, and drinking water. Duplicate
one-day diet samples were analyzed by Dr. Carol Weisskopf at the
Food and Environmental Quality Laboratory at Washington State
University. Nonetheless, more than half of the OP pesticides registered
in Washington State still fell outside these analytical capabilities.
Biological monitoring for multiple OP compounds is also challenging. Of the thirty pesticides used in Washington, for example, only five have compound-specific urinary metabolites. The lack of specific metabolites for OP pesticides led us to measure urinary dialkylphosphates--the common metabolites of the OPs. Six metabolic products are normally measured by gas chromatography following derivatization: dimethyl phosphate (DMP), dimethylthio phosphate (DMTP), dimethyldithio phosphate (DMDTP), diethyl phosphate (DEP), diethylthio phosphate (DETP), and diethyldithio phosphate (DEDTP). It is important to realize, though, that even this more generic assay does not necessarily capture all OP compounds. Eight of the thirty OP pesticides used in Washington are not measured with this technique.
The 1992 studies included soil and housedust sampling of forty-eight agricultural families and eleven reference families. Figure 1 provides median values for four OP pesticides in housedust and soil. These data indicated that housedust concentrations were substantially higher than soil concentrations for all compounds, and that the highest housedust concentrations were for azinphos-methyl and phosmet, both dimethyl compounds. These findings, coupled with knowledge that these children spent much of their time indoors, led to the conclusion that housedust concentration was the most useful indicator of exposure potential for this population. Figure 2 compares the OP pesticide housedust concentrations for agricultural and reference families, demonstrating that children in agricultural households had higher exposure potential than did children in reference families for all four OP compounds measured.
|
|
|
|
 |
 |
|
|
|
|
|
|
|
|
Our 1995 studies included housedust sampling in seventy-six homes
and collection of urine samples from 109 children. An initial
report of this study compared DMTP urinary concentrations of forty-eight
applicator children and eleven reference children. The patterns
for metabolite concentrations were similar to those for housedust
concentrations: about a four- to five-fold difference between
the groups.
Our studies in 1998 included biweekly urine samples from about fifty Wenatchee children for one year, samples from 100 children in two Seattle metropolitan area communities, and a pilot multi-pathway exposure analysis in thirteen homes. We are hoping to publish results for these studies sometime this year.

Translating the environmental and biological
measurements we have collected into a meaningful statement about
health risk has not been a simple task. First, we felt that the
parents of the children who participated in our studies deserved
clear and understandable feedback about the study results. The
letters we sent to parents included specific results for their
children, but also tried to answer the question, "Should
I be concerned about these levels from a health standpoint?"
We told parents that the levels we measured did not pose a serious
or immediate hazard to their children, and that exposures were
best described as "low level." We became convinced after
comparing our study results with available scientific information
that these children were not at risk for an acute health effect,
such as substantial decrease in their nervous system enzymes.
Yet when it comes to more subtle health effects, we don't have
a good answer. The jury is still out. A number of studies are
exploring the effects of low-level OP pesticide exposure on neurological
development in very young animals. New findings will be reported
periodically in the scientific literature, and will perhaps even
reach the newspapers. But it will be many years before the question
of long-term effects will be answered with any reasonable degree
of scientific certainty. In the meantime, what do we do?
Public health emphasizes prevention as the most effective means
of reducing risk. We have encouraged parents who wish to reduce
their children's exposure to adopt some commonsense procedures:
always follow pesticide label instructions, keep pesticides in
a safe place in the home, remove shoes and clothing that may have
pesticide residues before entering the home, and keep kids away
from pesticide-treated areas, both indoors and out. We have also
joined with scientists at the Fred Hutchinson Cancer Research
Center to develop a study in the lower Yakima Valley to see if
a community-based education program can reduce pesticide exposure
in children of agricultural workers.
The debate about pesticide health risks is likely to be a long and contentious one. The scientific uncertainty that has created the current risk information vacuum means that caution will be an important principle in regulation. In the meantime, good public health practice and common sense suggest we try to reduce our children's exposures wherever possible.
Dr. Richard Fenske is Professor of Environmental Health at the University of Washington's School of Public Health and Community Medicine, and Director of the Pacific Northwest Agricultural Safety and Health Center (PNASH). He serves on EPA's Science Review Board, a congressionally mandated advisory board for pesticide science policy. He can be reached at rfenske@u.washington.edu or (206) 616-1958.
|
|
Return to Table of Contents for the March 2000 issue
|
Return to Table of Contents for the March 2000 issue
Washington State University offers PRE-LICENSE courses (for those who do not have a license and need one) and RECERTIFICATION courses (for those who need to renew their current licenses). Fees are $35 per day if postmarked 14 days before the program, otherwise $50 per day. This fee DOES NOT include WSDA license test fee, which ranges from $25 to $170; for information on testing and fees, contact WSDA at (360) 902-2020 or http://www.wa.gov/agr/pmd/licensing/index.htm. Recertification courses offer 6 credits per day.
Ever wonder if following the rules is really worth it? In case your conscience and good stewardship aren't sufficient motivation, take a lesson from the adventures of Kap Dong Kim.
On January 24, 2000, Kim, owner of a ginger root farm in Hilo, Hawaii, was sentenced to four months in prison and a $5,000 fine in U.S. District Court in Honolulu. He was also ordered to pay $6,113 in restitution.
Kim previously pleaded guilty to illegally using the restricted-use pesticide nemacur on his ginger root crop in violation of the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). It seems he directed workers to apply it on the crop without following required standards for worker protection. One worker was poisoned and had to be hospitalized. When later questioned by a government official, Kim deliberately failed to disclose the pesticide application.
This case was investigated by the Environmental
Protection Agency's (EPA's) Criminal Investigation Division and
the Hawaii Department of Agriculture with the assistance of EPA's
National Enforcement Investigations Center, and was prosecuted
by the U.S. Department of Justice. Civil charges against Kim are
also pending.
Return to Table of Contents for the March 2000 issue
In reviewing the January postings in the Federal Register, we found the following item that may be of interest to the readers of Agrichemical and Environmental News.
In the January 6 Federal Register, EPA issued a cancellation order for cyanazine. Effective December 31, 1999, any distribution, sale, or use of cancelled cyanazine is only permitted as follows: Any cyanazine products that entered the channels of trade prior to December 31,1999, may be distributed, sold, and used through December 31, 2002. In Washington, cyanazine is registered for use on field corn seed crops, popcorn, sweet corn, and field corn as Bladex (DuPont), Cy-Pro (Griffin), and Extrazine (DuPont). (Page 771)
The PNN is operated by WSU's Pesticide Information Center for the Washington State Commission on Pesticide Registration. The system is designed to distribute pesticide registration and label change information to groups representing Washington's pesticide users. PNN notifications are now available on our web page. To review those sent out in the month two months prior to this issue's date, either access the PNN page via the Pesticide Information Center On-Line (PICOL) Main Page on URL http://picol.cahe.wsu.edu/ or directly via URL http://www.tricity.wsu.edu/~mantone/pl-newpnn.html. We hope that this new electronic format will be useful. Please let us know what you think by submitting comments via e-mail to Jane Thomas at jmthomas@tricity.wsu.edu.
| Chemical (type) | Federal Register | Tolerance (ppm) | Commodity (raw) |
|
||
|
|
|
Exp. Date | ||||
| mepiquat chloride |
01/12/00 page 1790 |
0.10 | grapes | No | N/A | N/A |
| 5.00 | raisins | |||||
|
spinosad (Factors A & D) (insecticide)
spinosad (Factors A & D) (insecticide) |
01/12/00 page 1802
01/12/00 page 1802 |
0.30 | apple |
No
No |
N/A
N/A |
N/A
N/A |
| 0.30 | barley | |||||
| 0.30 | animal feed (nongrass group) | |||||
| 0.02 | buckwheat grain | |||||
| 8.00 | cilantro leaves | |||||
| 0.02 | popcorn grain | |||||
| 0.02 | grass forage, fodder, and hay | |||||
| 1.00 | pearl and proso millet grain | |||||
| 0.02 | oat grain | |||||
| 0.02 | rye grain | |||||
| 10.00 | turnip greens | |||||
| 8.00 | watercress | |||||
| Comment: This is only a partial list of the tolerances established for spinosad on January 12. The list includes only those crops grown in the Pacific Northwest. | ||||||
|
bifenthrin (insecticide) |
01/25/00 page 3860 |
0.20 | grapes | Yes | New | 12/31/01 |
| Comment: This time limited tolerance is being established in response to EPA granting a Section 18 emergency exemption for the use of bifenthrin to control black vine weevils in Washington grapes. | ||||||
W.S.U. Pullman Home Page Comments and questions: cdaniels@tricity.wsu.edu Technical Assistance: scoates@tricity.wsu.edu Copyright © Washington State University / Disclaimer Electronic Publishing and Appropriate Use Policy University Information: 509/335-3564