
July 2002, Issue No. 195
A monthly report on environmental
and pesticide related issues
In This Issue
Upcoming
Conferences/Announcements
- New Publications
- Biotech
Crops
- Sustainable
Agriculture
- Organics
in Tree Fruits
- Educational
Opportunities
- Pesticide
Stewardship
- Direct
Seeding
- Health
& Safety
- IR-4 Input
Needed
- ...and more!
NOTE
ABOUT PRINTING A HARD COPY
Some AENews feature articles are available
in Portable Document Format (PDF) version, which is recommended for
printing. If you cannot read PDF
files, you may download Adobe Acrobat Reader free at http://www.adobe.com/prodindex/acrobat/readstep.html
If you choose to print from the HTML version you currently see on
your screen, you may need to set your browser's printing preferences
so that no margins are cropped.
|
||
|
Upcoming Conferences/Announcements
|
||
NOTE
ABOUT PRINTING A HARD COPY
Some AENews feature articles are available
in Portable Document Format (PDF) version, which is recommended for
printing. If you cannot read PDF
files, you may download Adobe Acrobat Reader free at http://www.adobe.com/prodindex/acrobat/readstep.html
If you choose to print from the HTML version you currently see on
your screen, you may need to set your browser's printing preferences
so that no margins are cropped.
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
"Pharm Farming"It's Not Your Father's Agriculture |
Click here for PDF version of this document (recommended for printing). Should you not have Adobe Acrobat Reader (required to read PDF files), this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html |
Dr. Allan S. Felsot, Environmental Toxicologist, Food and Environmental Quality Laboratory, WSU
Imagine a very wealthy country with unsurpassed expertise in discovering and successfully developing medications that cure some pretty nasty immune system diseases. Imagine not enough people being able to get the drug because manufacturers can’t make it fast enough. Well, you don’t have to use your imagination because it is happening today in the good old USA. “Biotech Industry Squeezed by Lack of ‘Breweries’,” screams a recent headline in the online version of the San Diego Union-Tribune newspaper. The article goes on to inform us that demand for protein-based drugs now on the market far exceeds industry’s ability to make enough product.
Before you jump to the conclusion that this is another story concocted by the evil pharmaceutical companies in an effort to rip off poor, unsuspecting consumers, let’s take a moment and look at how biotechnology has transformed the manufacturing process.
A Biotech Manufacturing Primer
If you associate pharmaceutical manufacturing with smoke-belching factories, think again. Many new pharmaceuticals are brewed like fine wines in fermentation vats lining very clean rooms. The “vats” are located in buildings covering acres of land and plumbed with miles of pipe. Imagine the energy and controls required to keep the vats humming at just the right temperature. And that’s just the beginning of the process. The stuff in the vat, known as the cell culture, has to be piped out and then extracted in another part of the factory.
As with any manufacturing process, production space is an issue. The cell cultures used to make the medicinal proteins can only produce so much during any given timeframe. If a company wants to brew more product, it needs to add more vats, requiring more space, more energy, and more personnel. Considering one of these “new age” clean plants costs an estimated $500 million dollars (Associated Press 2002), one can see why this industry might be pinched.
A Role for Agriculture
Fortunately for health care consumers, agricultural biotechnology may hold the solution to the pharmaceutical industry’s production problems. Instead of using gigantic space-gobbling, energy-intensive, expensive factories, companies are developing the ability to grow medicinal proteins in plants.
This isn’t about Uncle Ed putting in a few acres of specially bred plants out on the north forty. Nor is it about traditional agribusiness growing food plants containing pharmaceuticals (despite the Internet rumors). A select elite of meticulous growers will be the new “green” manufacturers of the next generation of medicinal proteins.
“Pharm farming,” my pet term for the growing of molecules with pharmaceutical applications in selected crops, is the third wave of agricultural biotechnology. And it is going to be an “industrial” process without physical walls but with scrupulous controls and regulatory oversight by at least three Federal agencies.
Riding the Third Wave
The first wave of agricultural biotechnology transformed plants to resists pests (e.g., Bt-corn and Bt-cotton that contain the insect toxic protein from Bacillus thuringiensis) or impart resistance to reduced risk herbicides like glyphosate (e.g., Roundup Ready soybeans). The second wave involves producing plants with quality-added characters that would increase agronomic efficiency (e.g., salt-tolerant tomatoes) or nutritional enhancements (e.g., high-lysine corn). The third wave has been given the epithet “plant molecular farming” and it refers to “the cultivation of plants for industrially, medically, or scientifically useful biomolecules, rather than for traditional uses of food, feed, or fibre” (Canadian Food Inspection Service 2001). With the exception of plant-based manufacturing of the enzyme trypsin (Van Brundt 2002), nothing has been commercialized at this point, but research and development is rapidly progressing, especially in the area of human medicines (Table 1).
TABLE 1 |
|
|
Potential
products under consideration for production via molecular farming
|
|
Primary Products |
Derived Products |
| Antibodies (immunoglobulins) | Bio-plastics |
| Enzymes (industrial, therapeutic, diagnostic, cosmetic) | Vitamins, co-factors |
| Structural Proteins (peptides, hormones) | Nutraceuticals |
| Antigens (vaccines) | Secondary Plant Metabolites (phenolics, glucosinolates, tannins, starches, sugars, fragrances, flavors, alkaloids) |
| Anti-disease agents, drugs | Fibers |
| Enzyme Inhibitors | |
| Note that the primary products are proteins. The derived products are mostly non-protein molecules that are synthesized by the plant if the correct enzyme systems to complete the metabolic pathway are present. | |
Proteins, CHOs, and PMPs
The products of pharm farming are called plant-made pharmaceuticals (PMPs). Presently, the substances under development are proteins with various functions. Proteins have been used for therapeutic purposes since the early 1980s when recombinant (i.e., genetically engineered) human insulin for injection was introduced to treat diabetes. Over the subsequent years, proteins were discovered with applications ranging from treatment of cancer and immune system diseases to hemophilia and hormone deficiencies (Vezina 2001).
The therapeutic proteins are produced in fermentation vats by transferring their coding genes to cell lines that have the ability to reproduce almost indefinitely (Figure 1). For example, one of the first commercial PMPs out of the block will likely be immunoglobulins (Ig’s), a catch-all term for different types of naturally occurring antibodies produced by mammalian plasma cells to ward off pathogens and their toxins. Ig’s are currently produced in fermentation cultures of Chinese hamster ovary (CHO) cells. CHO cells have been around since the late 1950s when they were taken from the ovaries of adult hamsters and induced to divide and replace themselves far beyond the typical 50-100 generations of cell cultures at the time. Today, specialized CHO cell lines can produce a wide variety of human proteins.
FIGURE 1 |
|
 |
 |
| The third wave of agricultural technology has the potential to replace expensive, energy intensive factories lined with stainless steel fermentation vats with higher yielding, lower cost, green factories without walls. (Picture of bioreactor from Vezina 2001). | |
CHO cells are commonly used to manufacture proteins because they divide rapidly and can be easily transformed to reproduce (i.e., replicate) and transcribe (i.e., read the code of) DNA from other organisms, including humans. Ig’s were particularly challenging because they are actually a combination of several protein chains that are linked together. Furthermore, they contain a very large sugar polymer called a glycan. Thus, Ig’s are known as glycoproteins. Several genes must work in concert to produce an intact Ig. However, success in overcoming the complexity of Ig assembly was reported over ten years ago (Wood et al. 1990). These early Ig-producing CHO cells could turn out 60 micrograms of antibody per one million cells every 48 hours.
Unfortunately, with current manufacturing capabilities, CHO cells just can’t keep up with the demand for protein products, especially the Ig’s. But over a decade ago, separate tobacco plants were transformed with mammalian genes that encoded separate component chains of an antibody (Hiatt et al. 1989). When individual plants containing the different chains were sexually crossed, the resulting progeny were able to synthesize a functional antibody. In the mid-1990s, the experiment was repeated successfully with a different kind of antibody (Ma et al. 1995). The third wave of agricultural biotechnology was building.
Plants on the Crest
The first wave of agricultural biotechnology showed plants could be easily and stably transformed with DNA from other species to produce useful traits that functioned reliably in the environment. So there was no reason that therapeutic proteins could not be grown in plants, as long as the genes could be found. While the first wave sought genes from bacteria like Bacillus thuringiensis (Bt), the therapeutic proteins of the third wave will require the source codes of human genes. Being proteins, Ig’s can be easily grown in plants. The DNA coding sequences that allow CHO cells to produce Ig’s can be modified to express well in plants. The plant readable gene modifications are moved to receptive plants cells along with accessory DNA pieces to give the plant the capability to start and end the process of transcribing the gene into its protein product.
Regardless of the nature or function of the protein, the process of transferring genes from one organism to another and allowing them functionality is basically the same among different species (see Carpenter et al. 2002 for an overview of the mechanics of producing biotechnology-derived crops). The basic technology of plant transformation has been well studied and commercially implemented in food crops like corn, soybean, wheat, and canola.
Although the therapeutic proteins can be expressed in any part of a plant, the goal for PMPs is to express the protein at the highest levels in the harvestable seed. Seeds are easier and more economical than whole plants to transport to a processing factory where the proteins can be extracted and purified in preparation for packaging. Furthermore, under controlled temperature conditions, seeds can be stored for prolonged periods without breaking down their protein content. Hundreds of acres of protein-containing seeds could inexpensively double the production of CHO cells in a fermentation factory.
As attractive as plants are for turning out great gobs of protein faster, cheaper, and more efficiently than CHO cells, their use raises all of the same concerns that have been expressed about the first wave of biotechnology-derived food crops. In particular, critics worry about potential gene flow to food crops of the same species, co-mingling of food and non-food crops, and worker exposure to plant material containing active pharmaceutical ingredients (APIs). One could argue that the benefits of pharmaceutical production in plants outweigh the risks, but industry has wholeheartedly embraced the precautionary principle to ensure that the risks of the third wave technology are minimized no matter how great the benefits (BIO 2002). (ED. NOTE: One definition of the precautionary principle comes from the 1998 Wingspread Conference in Racine, WI: "When an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause and effect relationships ar not fully established scientifically.")
Precautionary Principle at Work
Candidate plants for the production of PMPs will include familiar crops like alfalfa, canola, corn, potato, rice, safflower, soybean, and tobacco (BIO 2002). Although the leading candidates for transformation into workhorses of green manufacturing are familiar food and nonfood crops, they will be treated altogether differently than the biotechnology-derived crops designated for food markets. Regulations are swiftly evolving to ensure the utmost protection of food resources and the environment from meandering medicines. Specifically, the precautionary principle is hard at work in several areas to ensure the new technology is low risk and high benefit.
Current Regulatory Authority
The infrastructure of regulation has been in place for nearly a decade, and it continues to evolve as experience with biotechnology-derived food crops grows. Risk management must necessarily focus on providing protection for human health (worker and consumer) and ecosystems. This responsibility has been legislatively placed in the hands of four Federal agencies: USDA’s Animal and Plant Health Inspection Service (APHIS), the Food and Drug Administration (FDA), the Environmental Protection Agency (EPA), and the Occupational Safety and Health Administration (OSHA).
APHIS and the FDA will stand at the pinnacle of regulation over PMP production. APHIS will issue permits for growing PMPs during both the research and development phase and the production phase. Unlike the first wave crops, pharm crops will need perpetual permitting from APHIS. Permits for growing small acreages of pharm crops for development purposes are already being issued, and APHIS has published its mandates for ensuring maximum environmental protection (USDA-APHIS 2002).
FDA has domain over any products produced by pharm farming. The agency’s job is to ensure integrity (purity, correct dosages) and safety of the medicinal product. Before commercial production of the PMPs, FDA will have already ruled on the safety and efficacy of the pharmaceutical product. All pharmaceutical risk assessment testing will have to be conducted under Good Laboratory Practice (GLP) standards, similar to tests required by EPA for the registration of pesticides. GLP standards subject data to auditing, guard against fraud, and ensure that all submitted studies can be reconstructed from scratch.
FDA’s responsibility extends to the entire manufacture of the pharmaceutical, from production to waste streams, so its role necessarily will complement the role of APHIS because production on the farm will be the first step in the manufacturing process. To oversee production practices, FDA has developed regulations called GMPs (Good Manufacturing Practices), which are the manufacturing analog of GLPs. GMPs ensure consistent manufacturing processes and product safety, purity, and potency. As a system that documents practices in all stages of product manufacturing, GMPs will essentially spread out from its historical application within the walls of the factory to the wide-open spaces of the field.
EPA is commonly thought of having its dominion over agriculture through the regulation of pesticides. EPA would be initially involved in the regulatory oversight of PMPs if the plants contained pest-protection characters (like the Bt protein) or herbicide-tolerance characters that might require a new use pattern for a herbicide, and thus a pesticide product label change.
EPA also has responsibilities for protection of the environment from manufacturing processes through application of regulations under the Toxic Substances Control Act, the Clean Air Act, and the Clean Water Act. Thus, EPA does have regulatory options should pharm farming raise any environmental concerns not directly related to pest protection characters or pesticides. However, many of the environmental issues will have already been investigated and assessed by FDA as part of the review of manufacturing processes before full-scale production.
Finally, if worker safety becomes a concern owing to excessive exposure to PMPs during any stage of production, OSHA has responsibility to require practices that minimize risk.
Crop Knowledge
Development and subsequent testing of PMPs has proceeded mostly using corn and tobacco as the green factories. The list of candidate crop plants for PMP production (i.e., alfalfa, canola, corn, potato, rice, safflower, soybean, and tobacco) is no accident. In addition to accumulated experience in using biotechnology to endow these plants with new traits, mountains of information are known about their physiology and ecology. Candidate pharmaceutical producing plants have been studied with respect to pollination, genetics, seed dormancy, and weediness potential. This information is useful for addressing several concerns, including pollen movement and subsequent gene flow between conventionally bred and biotechnology-derived crops. A long history of cultivation shows that the candidate crops are the least likely to be invasive of “natural” ecosystems. All of this information will be used to ensure maximal isolation of the plants from food producing crops.
Principles of Confinement
Long before commercial utilization of crops for synthesis of PMPs, regulatory agencies will have refined rules for implementing the most important operating practice for safe manufacturing: the Principles of Confinement. Confinement essentially means keeping the crop and its products on the land where it was grown until removed for processing, with no inadvertent exposure to the public and minimal exposure of products to workers and the environment. Effectively confined pharm crops will conform to the following principles that have been elucidated by the biotechnology companies through the Biotechnology Industry Organization (BIO 2002):
- Prevention of inadvertent
human exposure to PMPs through food and feed
- Minimized occupational and
environmental exposure to PMPs during ALL phases of production
- Rigorous compliance with
confinement measures
- Analytical methods for detection
of expression (i.e., protein) products
- Full cooperation with regulatory
reviews of confinement measures and on-site inspections
- All confinement systems and procedures must be based on sound scientific principles
Identity Preservation: A Closed Loop System
Preventing co-mingling of pharm crops with food crops will be a prime directive for industry as well as regulatory agencies. The misadventures over the co-mingling with food corn of the non-food Bt-corn hybrid known as StarLink®, which was only registered for animal feed, will not be repeated. Precaution demands that Standard Operating Practices (SOPs) be implemented for a functional identity preservation system. Such a system ensures that the pharm crop is completely segregated from all other crops and that protocols are in place for production and handling of the crop. Achieving this goal is possible with implementation of chain-of-custody procedures that track the product through every stage of production and processing.
With an effective chain-of-custody program, the crop and its products are never out of sight. At every step of crop production, commodity transportation, and product handling, someone acknowledges in writing that all procedures have been carried out in compliance with the SOPs. In short, a completely closed loop identity preservation system not only protects the quality and purity of the final protein product, but it complements confinement to ensure maximal environmental and worker protection.
SOPs for Confinement Systems
BIO has recently released a white paper that is functionally a reference document for confinement and development of PMPs (BIO 2002). SOPs for each specific pharm crop must be developed and implemented to meet the Principles of Confinement. Pertinently, the SOPs apply to research and development of pharm crops in addition to commercial production. Effective confinement SOPs will address these elements:
Training
All growers and other individuals involved in development and production of PMPs must be trained to carry out the principles of confinement.
Contracts and Channeling
Seed for PMPs must not be sold through conventional channels and will be available only to trained contract growers. All processing of crop product must be conducted in complete isolation from commercial food and feed channels.
Site Selection and Security
Field-testing and production sites must be selected to meet the confinement measures most appropriate for the particular pharm crop. For example, regulatory-mandated distances of separation of pharm crops and food crops must be rigorously enforced. Security measures may be necessary to provide control of site access and exposure to humans or wildlife.
Crop Production
Crop production will be considered to include all procedures associated with seed production, planting, growing, harvesting, transportation, and storage of the pharm crop. A variety of physical, biological, and temporal procedures must be employed to limit environmental and worker exposure to a specific crop or expression product.
Identification
Pharm crops and plant material from that crop must be distinguishable to the developer from any other crop through clear identification methods during all production phases.
Containers
All containers and packing materials used for shipment, transportation, and storage must have integrity, be properly labeled, and possess the ability to be thoroughly cleaned or disposed of.
Equipment
Any equipment used in any phase of crop production and initial processing of the PMP must be dedicated to the specific product or thoroughly cleaned prior to use with any other crops.
Disposition of Plant Material
All unused plant material, both on farm and off, must be disposed of in a manner (designated by regulations) that prevents inadvertent exposure and co-mingling with plant material intended for food or feed.
Verification
All adherence to confinement SOPs must be verified through a system of documentation and record keeping.
Compliance Assessment
The entire production system must be subject to appropriately timed internal and external reviews and inspections to ensure compliance with all SOPs.
Monitoring
During and after harvest, field trial and production sites must be monitored for any unusual occurrence, including deleterious effects on plants, non-target organisms, or the environment. All such occurrences must be reported to the appropriate regulatory agency. Sites must be monitored to ensure that all plant material stays at the site until disposed of according to SOPs. After harvest, fields must be monitored for new emergences of volunteer crops.
Remediation
Remedial plans must be in place to employ procedures to mitigate any potential effects if the confinement system does not achieve its desired results. Furthermore, confinement systems need to be modified as needed to improve performance and ensure adherence to the over-riding confinement and identity preservation-closed loop principles.
Regulatory Footprints
Judging from current controversies over cross pollination between biotechnology-derived and conventionally bred crops, gene flow from pharm farms is likely to be the most contentious issue during the crop production phase of PMP technology. Concerns are already being addressed by industry, APHIS, and the Canadian Food Inspection Service (BIO 2002, USDA APHIS 2002, CFIS 2001); the main ones revolve around pollination of food crops and subsequent inadvertent setting of seed containing the active pharmaceutical ingredient (API).
During the permitting process
for production of pharm crop seeds for research, APHIS has already imposed
strict rules on the separation distances from food crops and the types
of plants that can be grown to form a physical barrier to trap migrating
pollen (USDA APHIS 2002).
For example, the following APHIS rules apply to all protocols for field
testing of corn for production of PMPs (USDA APHIS 2002).
- Applicants must plant the
transgenic corn at sites that are at least one mile away from corn seed
production (e.g., breeder, foundation, certified, and registered).
- Applicants must ensure that
any corn from previous seasons is harvested and removed in a radius
of 0.25 miles of the transgenic corn lot before the transgenic corn
is sown.
- The land within 25 feet
of the transgenic plant area must remain fallow during the test.
- If no buffer crops are
grown, then applicants must ensure that no other corn plants are grown
within a radius of 0.5 miles of the transgenic test plants at any time
during the field test. Applicants must plant their corn no fewer than
21 days before or 21 days after the planting dates of any other corn
that is growing within a zone extending from 0.5-1.0 mile of the test
plants.
- If buffers are used, applicants
must ensure that no corn plants other than those of the buffer strip
are grown within a 0.25 mile-radius of the transgenic test plants at
any time during the field test. A buffer strip consisting of 6 rows
of male-fertile, nontransgenic corn must be planted just inside the
0.25-mile perimeter distance. The buffer strip plants must be treated
with the same confinement and disposal measures that are used for the
transgenic crop.
- No tests in 2003 or thereafter will be permitted to use corn buffer rows. This proscription will reduce the chances of co-mingling of pharm crops with food crops.
The USDA APHIS did not pluck these rules for corn out of the thin air. Foundation-certified corn seed requires isolated distances between cornfields of 660 feet (only ~1/8 of a mile) to protect the hybrids from cross-pollination. Thus, APHIS is being quite conservative in applying separation distances from test plots and other crop fields. Of course, the separation distances will vary depending on the specific crop and known distances of pollen travel.
Validating the Principles of Confinement
Recall that one of the principles of confinement is that all practices must be scientifically valid. Academic researchers have spent little time over the last thirty years studying pollen travel and gene flow for our most-farmed crops (e.g., corn, soybean, wheat). Under the precautionary principle, the advocate for a technology is responsible to ensure its safety. Therefore, industry is obligated to step in to figure out whether the APHIS-proposed guidelines are sufficient. Exercising good faith and judgment, industry itself, as well as university researchers, are already testing the adequacy of confinement principles to prevent gene flow between food and non-food crops.
Two papers that will be presented at the Fall 2002 meetings of the American Society of Agronomy (ASA) will show data confirming that suggested buffer areas and required separation distances are adequate to keep pollen “on the farm,” so to speak (Stevens et al. 2002; Halsey et al. 2002). Dr. Gene Stevens from the University of Missouri was generous enough to share with me the experimental design for testing separation distance hypotheses (Figure 2) and also some of his data that he will present to the scientific community at the ASA meeting.
To make a long story short, Dr. Stevens first explained how one of the candidate PMP technologies intended for Ig production will work during production. Corn produces separate male and female flowers (respectively called tassels and silks). Three to five days before a tassel is visible on a corn plant, it can be found tightly rolled in a whorl of new leaves near the top of the stalk. The immature tassels along with two or three leaves can be removed from the plant in a process called detasseling to create the equivalent of a female plant.
Basically, female corn lines (i.e., plants that have been detasseled) containing the genes for the Ig will be grown in four rows that alternate with four rows of male corn (corn with intact tassels) without the Ig genes. Because all female plants are detassled, they are incapable of producing pollen containing the Ig gene. However, their ovules (the future seeds) will contain the Ig gene. The female plants will be allowed to receive pollen from the four rows of male corn without the Ig genes. After fertilization, the resulting seeds will contain the right complement of genes to make a functional Ig. Creating a plant with only maternally inherited PMP genes combined with detasseling is the best production system for preventing gene flow.
Detasseling has been a common practice in corn hybrid seed production for many years. Although it is highly effective procedure, tassels are occasionally missed. Most seed certification inspectors allow less than 1% detasseling error. Regulatory agencies need to know what kinds of separation distances must be maintained at different levels of detasseling efficiency to avoid gene flow to other corn fields. To answer this question, Dr. Stevens planted yellow-kernel inbred corn in a 10-acre block in three 160-acre cotton and bean fields in southeastern Missouri (Figure 2). The block served as a source of fertile pollen to detect in white corn strips planted in other parts of the field.
FIGURE 2 |
 |
| Schematic plot design for experiments conducted at three locations in Missouri to determine the extent of gene flow from a biotechnology-derived corn crop. Yellow and white corn was planted in alternating four-row strips in the center pollen source block. Fallow ground was left around the pollen source area and sterile male yellow corn was planted around the fallow area. White corn in 12-row strips was planted at 330, 660, and 900 feet beyond the edge of the pollen source corn block. Several rows of fallow were left around the white corn strips. The rest of the field was planted to either beans or cotton depending on the location of the field. Individual kernels on corn cobs collected from pre-selected areas in each of the white corn trap strips were sampled to determine gene flow from the yellow corn source block. (All information was obtained from Dr. Gene Stevens, University of Missouri.) |
Within the 10-acre corn pollen blocks, four rows of yellow-kernel females were planted in an alternating pattern with four rows of white-kernel males. When the yellow female rows were detasseled by Dr. Stevens’ research team, some of the plants were intentionally missed. The levels of detasseling were 0%, 80%, 90%, and 100% of the total plants in each row. For each detasseling treatment in the pollen block, a different yellow inbred cultivar was planted which contained a specific transgenic trait to use as a tracer.
To aid in pollen containment, the pollen source block was surrounded by 10 feet of fallow ground and then 12 rows of male sterile corn. The rest of the field was planted to either beans or cotton, but at distances of 330, 660, and 900 feet from the pollen block, four-row strips of a white corn hybrid were planted on three different planting dates.
Yellow corn seed color is dominant over white seed color. Therefore, any yellow kernels found in the white hybrid strips were fertilized by pollen that came from yellow corn in the central pollen block (Figure 2). The researchers used a molecular analytical technique known as PCR (polymerase chain reaction) to detect each of the specific transgenes used as tracers in the different yellow inbred corn cultivars. Using this tracer system, any yellow corn in the white corn strips could be traced back to pollen from a specific detasseling treatment.
When the corn seeds matured, Dr. Stevens’ team pored over thousands of ears of corn in the white hybrid strip looking for yellow seeds. They expressed their results as the percentage of yellow kernels among the white kernels in the corn planted at two different distances from the center block (660 and 900 feet). The research team noted that the planting date of the white hybrid corn strips had a large effect on gene flow (i.e., on detection rates of yellow kernels). This observation means that a narrow window of time existed when the viable pollen produced by the yellow corn was in synchrony with the receptive silks on the white hybrid ears.
The greatest amount of gene flow, as represented by findings of yellow corn kernels on the white corn cobs, occurred in the northern section of white corn located 660 feet from the pollen block and was associated with pollen from corn with no detasseling. The incidence of yellow kernels was 0.0301%. Gene flow was probably comparatively greater in the northerly most corn strips because the prevailing wind was from the southwest.
The amount of gene flow dropped as the levels of detasseling and isolation distance increased. At 900 feet, which is a shorter distance than required in the APHIS regulations for separation of PMP corn and other cultivars, the incidence of yellow kernels in the white corn was 0.0013% from the 90% detasseled corn rows. When 100% of the corn was detasseled, no yellow kernels were detected on the white corn cobs.
To put the probability of finding a kernel fertilized from yellow corn pollen into perspective, when no detasseling had occurred, 3 out of every 10,000 seeds at the 660 foot distance from the center block had yellow seeds. When 90% of the yellow corn was detasseled, only 1.3 seeds out of every 100,000 seeds were yellow in the strips located 900 feet from the pollen source block. If an average ear of corn contains 500 seeds, then the significance of cross-pollination at the 900-foot distance would be one seed containing a hypothetical pharmaceutical protein for every 150 ears. Very similar results have been obtained in studies conducted in California and Washington State (Halsey et al., 2002).
In essence, the data generated so far on gene flow potential support the APHIS regulatory requirements for separation distances between pharm crops and food crops. Pertinently, the minimum separation distances required by APHIS are significantly longer than the distances shown to have almost no gene flow in the Missouri, California, and Washington State experiments. Of course, one kernel among tens of thousands of kernels can still pose a worry, so the precautionary principle rightly asks what the consequence to people or the environment would be to such a low exposure to an active pharmaceutical ingredient (API).
When Confinement Goes Wild
So what would the consequences to humans and wildlife be should some wayward pollen land on a food crop and produce a few seeds with APIs? And, while we’re dreaming up hazard scenarios, let’s also ponder the effects that APIs in the non-harvested plant material might have on ecological integrity of the agricultural field or nearby uncultivated land.
As illustrated thus far in the experiments to test confinement strategies, even when cross-pollination in corn has occurred, the probability of finding one seed in thousands of seeds is pretty low. From this incidence, we can conclude that under proper confinement measures, any inadvertent exposures attributable to gene flow are very low, and thus the risk of adverse effects are correspondingly low.
But let’s say a person (or a bird or mouse) is inadvertently exposed through their food to an API. Fortunately, the APIs under development are proteins, and we know a lot about the bioavailability and fate of proteins in the environment and in organisms. The candidate proteins under development all occur naturally in animals, including humans. Indeed, some of the therapeutic proteins are actually coded for using human gene constructs. All proteins can be tested for digestibility in the stomach or intestine. For example, Ig’s are already known to be rapidly digestible; for that reason, therapeutic doses are normally administered by injection or intravenously rather than orally. When we eat meat, we eat non-therapeutic doses of Ig’s.
All organisms, including soil bacteria and fungi, contain protein-degrading protease enzymes. Once the plant hits the ground, a farmer can disk it into the soil and, with a little moisture, any proteins, including the pharmaceuticals, will degrade to harmless amino acids. Even when the proteins stick to clay particles, as has been observed for the Bt toxin protein (Stotzky 2000), they are not biologically available nor are they significantly mobile, especially under natural moisture conditions (Carpenter et al. 2002).
One pertinent point to consider when pondering ecological effects of PMP technology is that for any one product, very limited acreage will be used. For example, about 1000 acres may be required to produce enough Ig’s of any kind. However, that acreage will not be placed in one area. Ideally, several states will be chosen that have very little of the food crop counterpart in production. Thus, not only will the acreage be more manageable owing to a limitation in size in any one location, but the specific location itself will be comparatively devoid of the food crops subject to cross pollination.
Life on the Pharm Farm
The research and development of PMPs is moving very swiftly. But no one should retain the idea that the technology has not been well tested first in the laboratory. For example, over the last ten years, a swelling body of scientific literature developed to show that therapeutic animal proteins could be expressed in plants. The extracted proteins retained their function when given to animals (Hiatt et al. 1989; Mason et al. 1992, 1996; Haq et al. 1995; Ma et al. 1995, 1997, 1998; Thanavala et al. 1995; Miele 1997; Arakawa et al. 1998; Tackett et al. 1998; Lerouge et al. 2000; and other recent references cited in Kirk 2001). Thus, we know the biochemical part of the technology works and we know a lot about the identity of the proteins and their safety. Besides, they won’t be approved by the FDA unless they are proven safe using the same level of scrutiny given to all synthetic pharmaceuticals.
Manufacturing the protein in factories without walls is not even a new concept considering that humans have been using medicinal plants for ages. These non-biotechnology-derived medicinal plants must be grown and harvested and extracted in a manner that ensures the integrity and safety of the medicine. However, forceful insertion of medicinal traits into plants using biotechnology makes some people nervous. But we are not talking about “novel” proteins. The therapeutic proteins are the same as those already in our body. Most of the proteins have already been produced as medicines using CHO cell fermentation. They’re well characterized and have been through safety assessments and often human clinical trials. The manufacturing process is the real novelty, but it will be regulated stringently as if the protein was being manufactured in a factory.
Some opponents of biotechnology applied to crops may complain that one of the principles of confinement will essentially be a self-policing provision to ensure all SOPs are verifiably implemented. However, if these opponents examine organic agricultural practices, they will find a self-policing system of certification that works quite well. Although some state agricultural agencies are engaged in certification, much is based on private certifiers and self-reported practices combined with inspections. Production of foundation seed also relies on an industry-policed system. Thus, specialty crop production practices have always been self-policed, but PMP production will have the additional safeguard of being overseen by at least three regulatory agencies.
Few acres will be needed to grow any one PMP, but the acres that are used and the resulting crop will be the subjects of extraordinary scrutiny throughout the whole production and post-production process. Only elite growers who commit wholeheartedly to the principles of confinement and identity preservation need apply. They will be duly rewarded for their technical skills, knowledge, and infrastructure, but life on the pharm farm will never be the same as in the good old days on Uncle Ed’s north forty.
Dr. Allan Felsot is an Environmental
Toxicologist with the Food and Environmental Quality Laboratory at Washington
State University. He can be reached on the university's Tri-Cities campus
at afelsot@tricity.wsu.edu or (509) 372-7365. Dr. Felsot is a frequent
contributor to AENews and co-author of the recently released Comparative
Environmental Impacts of Biotechnology-Derived and Traditional Soybean,
Corn, and Cotton Crops from the Council
for Agricultural Science and Technology, http://www.cast-science.org.
REFERENCES
Arakawa, T., J. Yu, D. K. Chong, J. Hough, P. C. Engen, and W. H. Langridge. 1998. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nature Biotechnology 16(10):934-938. (GO BACK)
Associated Press. 2002. Biotech industry squeezed by lack of 'breweries'. San Diego Union Tribune, May 28, 2002. (http://www.union-tribune.com/news/science/20020527-1423-bioreactorshortage.html). (GO BACK)
Biotechnology Industry Organization (BIO). 2002. Reference document for confinement and development of plant-made pharmaceuticals in the United States. http://www.BIO.ORG/. (GO BACK)
Canadian Food Inspection Service (CFSI). 2001. Plant molecular farming--discussion document. http://www.inspection.gc.ca/english/plaveg/pbo/mainplnt_mfe.shtml. (GO BACK)
Carpenter, J., A. Felsot, T. Goode, M. Hammig, D. Onstad, and S. Sankula. 2002. Comparative Environmental Impacts of Biotechnology-derived and Traditional Soybean, Corn, and Cotton Crops. Council for Agricultural Science and Technology, Ames, Iowa. http://www.cast-science.org. (GO BACK)
Hiatt, A., R. Cafferkey, and K. Bowdish. 1989. Production of antibodies in transgenic plants. Nature 342(6245):76-78. (GO BACK)
Halsey, M. E., F. G. Gaitan-Gaitan, K. M. Remund, and S. A. Berberich. 2002. Polllen-mediated gene flow in maize as influenced by time and distance. Abstract & poster to be presented at the Annual Meeting of the American Society of Agronomy, November 2002. (GO BACK)
Haq, T. A., H. S. Mason, and C. J. Arntzen Clements. 1995. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268:714-716. (GO BACK)
Kirk, D. A. 2001. Potential impacts of plant molecular farming on biodiversity. Report prepared for the Canadian Food Inspection Service http://www.inspection.gc.ca/english/plaveg/pbo/mf/mf_kirke.pdf . (GO BACK)
Lerouge, P., M. Bardor, S. Pagny, V. Gomord, and L. Faye. 2000. N-glycosylation of recombinant pharmaceutical glycoproteins produced in transgenic plants: towards an humanisation of plant N-glycans. Current Pharmaceutical Biotechnoloy 1(4):347-354. (GO BACK)
Ma, J. K. C., A. Hiatt, M. Hein, N. D. Vine, F. Wang, P. Stabila, C. van Dolleweerd, K. Mostov, and T. Lehner. 1995. Generation and assembly of secretory antibodies antibodies in plants. Science 268:716-719. (GO BACK)
Ma, S.-W., D.-L. Zho, Z.-Q. Yin, R. Mukherjee, B. Singh, H.-Y. quin, C. R. Stiler, and A. M. Jevnikar. 1997. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nature Medicine 3(7):793-796.
Ma, J. K. C., B. Y. Hikmat, K. Wycoff, N. D. Vine, D. Chargelegue, L. Yu, M. B. Hein, and T. Lehner. 1998. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nature Medicine 4(5):601-606.
Mason, H. S., D. M. K. Lam, and C. J. Arntzen. 1992. Expression of hepatitis B surface antigen in transgenic plants. Proc. National Academy of Sciences 89(December):11745-11749. (GO BACK)
Mason, H., J. M. Ball, J.-J. Shi, X. Jiang, M. K. Estes, and C. J. Arntzen. 1996. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. National Academy of Sciences 93(May):5335-5340.
Miele, L. 1997. Plants as bioreactors for biopharmaceuticals: regulatory considerations. Trends in Biotechnology 15: 45-50. (GO BACK)
Tackett, C. O., H. S. Mason, G. Losonsky, J. D. Clements, M. M. Levine, and C. J. Arntzen. 1998. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nature Medicine 4(5):607-609. (GO BACK)
Thanavala, Y., Y.-F. Yang, P. Lyons, H. S. Mason, and C. Arntzen. 1995. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc. National Academy of Sciences 92(April):3358-3361. (GO BACK)
Stevens, W., S. Berberich, C. Wiltse, M. Horak, M. Halsey, K. Remund, A. Sheckel, D. Dunn. 2002. Gene flow studies to optimize containment of regulated products produced in corn. Paper to be presented at the Annual Meeting of the American Society of Agronomy, November 2002. (GO BACK)
Stotzky G. 2000. Persistence and biological activity in soil of insecticidal proteins from Bacillus thuringiensis and of bacterial DNA bound on clays and humic acids. Journal of Environmental Quality 29:691-705. (GO BACK)
USDA Animal & Plant Health Inspection Service (APHIS). 2002. Information of field testing of pharmaceutical plants in 2002. http://www.aphis.usda.gov/ppq/biotech/ . (GO BACK)
Van Brundt, J. 2002. Molecular farming’s factories. Signals Magazine (http://www.signalsmag.com/signalsmag.nsf/0/3764D93A977219F888256B640069E224) (GO BACK)
Vezina, L. P. 2001. Plant-made pharmaceuticals: a new opportunity for Canada. Candian Food Inspection Service Forum on Plant Molecular Farming (presentation available on the CFIS web site http://www.inspection.gc.ca/english/plaveg/pbo/mf_fore.shtml) (GO BACK)
Wood, C. R., A. J. Dorner, G. E. Morris, E. M. Alderman, D. Wilson, R. M. O'Hara Jr., and R. J. Kaufman. 1990. High level synthesis of immunnoglobulilns in Chinese hamster ovary cells. J. Immunology 145(9):3011-3016. (GO BACK)
Return to Table of Contents for the July 2002 issue
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
A Good Year for the LadiesLadybird Beetles in Eastern Washington |
Click here for PDF version of this document (recommended for printing). Should you not have Adobe Acrobat Reader (required to read PDF files), this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html |
Dr. David G. James, Entomologist, WSU
 Ladybird
beetles, also known colloquially as “ladybugs,” are one of our
most valuable insect friends. Year after year, these “ladies”
kill untold numbers of aphids, mealybugs, scale insects, and mites on
crops and ornamentals.
Ladybird
beetles, also known colloquially as “ladybugs,” are one of our
most valuable insect friends. Year after year, these “ladies”
kill untold numbers of aphids, mealybugs, scale insects, and mites on
crops and ornamentals.
The Virgin Mary and her Seven Joys and Seven Sorrows
People have revered ladybugs since at least the Middle Ages, recognizing their value as biological control agents. They are known by at least 329 names in no fewer than 55 languages. Many of the names relate to God or the Virgin Mary. “Ladybird” derived from “our Lady’s bird,” a reference to Mary, and was originally applied to the seven-spotted English version of the insect. The red base color represented Mary’s cloak and the seven black spots represented her seven joys and seven sorrows. In Denmark, ladybugs are called “Mary’s hens,” while in France they are known as “cows of the Virgin.” The Cherokee name for them means “great beloved woman.”
A Good Year for the Ladies
 Spring
2002 was notable in eastern Washington for a great abundance of ladybugs.
Large numbers of these familiar red or orange beetles with black spots
were seen in orchards, vineyards, and field crops as well as in residential
backyards. They appeared to build up in March and April on a good supply
of aphids that thrived under cool, moist conditions, and became very noticeable
during May and June. There are thousands of species of ladybugs worldwide,
but until recently two species, the transverse ladybug and the convergent
ladybug, were the ones most commonly found in central Washington orchards,
fields, and gardens. Both are native to the western United States. The
convergent ladybug is available from suppliers of beneficial insects for
home garden and crop use.
Spring
2002 was notable in eastern Washington for a great abundance of ladybugs.
Large numbers of these familiar red or orange beetles with black spots
were seen in orchards, vineyards, and field crops as well as in residential
backyards. They appeared to build up in March and April on a good supply
of aphids that thrived under cool, moist conditions, and became very noticeable
during May and June. There are thousands of species of ladybugs worldwide,
but until recently two species, the transverse ladybug and the convergent
ladybug, were the ones most commonly found in central Washington orchards,
fields, and gardens. Both are native to the western United States. The
convergent ladybug is available from suppliers of beneficial insects for
home garden and crop use.
Exotic Ladies
In recent years, a new ladybug, the multi-colored Asian ladybeetle, has become increasingly common in our region, providing good biological control of aphids in some crops like hops. This species was introduced by the USDA into the eastern United States during the 1960s, ‘70s, and ‘80s for control of forest and orchard aphids. Fourteen thousand Asian ladybeetles were released by USDA near Yakima in 1980. For many years the beetle failed to establish in any of its intentionally released zones, but during the late eighties and nineties large populations developed in many eastern states. Large populations also began developing about that same time in the coastal areas of Washington and Oregon. The Asian ladybeetle was rarely seen in eastern Washington until the late nineties, but was common in 2001 and again this year.
Ladies Behaving Badly
 In
their native Asian habitat, these ladybugs gather in large numbers during
autumn on elevated, rocky outcrops prior to overwintering en masse in
nearby protected situations (e.g., under rocks, cliff ledges, caves).
In the urban environment, Asian ladybeetles use light-colored buildings,
walls, signs, and similar structures as their gathering places and they
often end up overwintering in buildings including homes. As much as we
in agriculture appreciate the “ladies,” when hundreds or thousands
of them overwinter in someone’s home, it causes a considerable nuisance.
This has proven to be a problem in many eastern U.S. states.
In
their native Asian habitat, these ladybugs gather in large numbers during
autumn on elevated, rocky outcrops prior to overwintering en masse in
nearby protected situations (e.g., under rocks, cliff ledges, caves).
In the urban environment, Asian ladybeetles use light-colored buildings,
walls, signs, and similar structures as their gathering places and they
often end up overwintering in buildings including homes. As much as we
in agriculture appreciate the “ladies,” when hundreds or thousands
of them overwinter in someone’s home, it causes a considerable nuisance.
This has proven to be a problem in many eastern U.S. states.
In another instance of bad behavior, Asian ladybeetles have begun infesting ripening grapes in Ohio, New York, Pennsylvania and Indiana. In large numbers, the beetles cause tainting and odor problems in wine, presenting what could become a serious concern to grape growers.
Watching for Exotic Ladies in Eastern Washington
The recent increase in Asian ladybeetle abundance in eastern Washington may be a consequence of a series of milder winters or some other climatic or environmental factor. Numbers may return to the previous low levels when conditions alter. However, it is also possible that the beetle has adapted better to eastern Washington conditions and will maintain or even increase populations further in the future. This will undoubtedly enhance biological control of aphids in orchards, on trees, and in some crops such as hops. But as we have seen in the east, it may also develop into an urban and possibly a vineyard problem.
I believe the arid climate and environment of eastern Washington is sufficiently sub-optimal to prevent the population explosions of Asian ladybeetles seen in the east. Nevertheless, we should remain vigilant and aware of the possible threat that this ladybeetle poses to our rapidly expanding viticultural industry.
Because of this concern, we ask that readers report any large aggregations of ladybeetles on or in buildings or on grapes so we can monitor them. Phone or e-mail any such sightings to me, David James, at (509) 786-9280 or djames@tricity.wsu.edu.
 An
English Lady in Washington
An
English Lady in Washington
The Asian ladybeetle is not the only new ladybug in eastern Washington. The English seven-spotted ladybird (of Virgin Mary fame) is also now a common part of our ladybug fauna. Like the Asian species, the seven-spotted ladybird was introduced into the United States by the USDA and has gradually spread throughout the country, turning up in Washington in 1990. However, 2002 appears to be the first year that its numbers have increased so much that during spring it was the dominant species in crops and ornamentals in the lower Yakima Valley. Fortunately, this species has not exhibited the nuisance behaviors of the Asian species. It does not use houses as overwintering sites and has not been recorded as a contaminant problem on grapes. It is a very effective predator of aphids, scale insects, and other pests and it will strengthen our biological control programs.
Will the Exotic Ladies Displace Native Ladies?
That is the six-billion-dollar question! We have no ready answer yet, although evidence is accumulating from various parts of the country suggesting that this may be the case. It will probably depend on how well the exotics adapt to different environments. In some areas they may totally displace native ladybugs, but in others, the natives may always be better adapted and thus repel the exotics.
David James is an entomologist at WSU’s Irrigated Agriculture Research and Extension Center (IAREC) in Prosser. He can be reached at (509) 786-9280 or djames@tricity.wsu.edu.
Return to Table of Contents for the July 2002 issue
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
Organic Farming Continues to Expand |
Click here for PDF version of this document (recommended for printing). Should you not have Adobe Acrobat Reader (required to read PDF files), this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html |
David Granatstein, Sustainable Agriculture Specialist, WSU Center for Sustaining Agriculture and Natural Resources
When I worked on an organic farm in Okanogan County during the 1970s, organic farming represented a very small sliver of American agriculture. It was not clearly defined beyond the preference for natural techniques and materials and the avoidance of synthetic pesticides and fertilizers. Soil and its organic matter were central concerns. Information on organic production was limited and often anecdotal. All of this relegated organic farmers to the fringe of Washington agriculture.
Mainstreaming Organics
Much has changed in the ensuing decades. Organic farming and processing methods have been codified by laws and through various certification organizations. In 1985, the Washington State Legislature passed the Organic Food Products Act, which led to the establishment of the Organic Food Program. This program, begun in 1988 within the Washington State Department of Agriculture, certifies organic products within Washington State. Across the country, demand for organic foods grew steadily during the 1980s and accelerated to a 20-30% increase per year during the 1990s. This market growth was accompanied by an increase in organic farm acreage nationally.
Washington State experienced more than a six-fold increase in organic acreage in less than a decade (Fig. 1). Organic farming has proven to be a biologically viable approach to farming and in many cases a more profitable one for growers. I remember when Earl Butz, former U.S. Secretary of Agriculture, asked, “Which half of the world will starve if we switch to organic farming?” It looks like that was the wrong question.
FIGURE 1 |
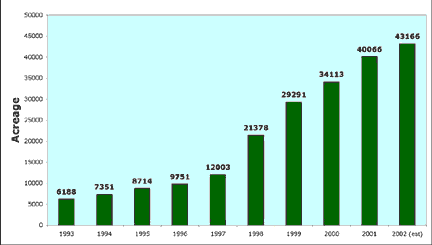 |
|
Historical change in organic farming acres (certified and transitional) in Washington State. |
With expanded production, organic agriculture has moved into the mainstream. Many farms and food businesses ventured into organic to test the waters and to diversify their markets. Organic farms are no longer primarily small acreage, labor-intensive operations run by people with a particular philosophical view of agriculture. Large-scale, highly mechanized organic farms are present in Washington and have increased the demand for products, support, and services (e.g., research, extension, promotion, marketing).
Washington’s Organic Sector
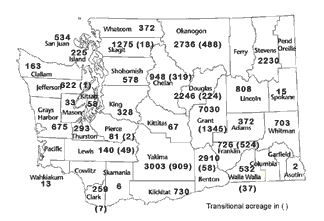
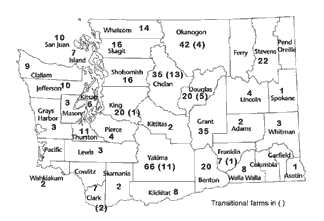
Growers and others in the organic sector have indicated the need for a publicly available picture of the changes in organic farming. Since Washington State Agricultural Statistics Service currently does not break out organic farming as a separate category, I recently compiled some statistics that illustrate the status of organic farming today. Figure 2 portrays the reported acreage of organic farms by county as of July 2001, while Figure 3 shows the number of farms with certified land or in transition status. These numbers only reflect those farms certified by the WSDA Organic Food Program. While this program conducts nearly all the certification within the state, it does not include exempt growers (sales <$5000/year) or growers that do not rely on certification for their local, direct marketing.
The majority of organic production in the state is located in the irrigated areas of central Washington. Grant County has the largest amount of certified acreage, while Yakima County has the largest number of certified organic growers. Organic farming is thriving in western Washington as well. In 1988 there were 33 certified organic farms in western Washington; today there are 142. Acreage is smaller in western Washington, but an expanding number of farmers are doing quite well there, many utilizing direct marketing outlets.
FIGURE 2 |
FIGURE 3 |
 |
 |
|
Estimated
organic farm acreage by county, July 2001.
|
Estimated number of organic farms by county, July 2001. |
Which Crops Are Going Organic?
The proportion of certified organic
acreage in the state by crop type is illustrated in Figure 4, with vegetables,
tree fruit, herbs, and forages as the leading segments.
FIGURE 4 |
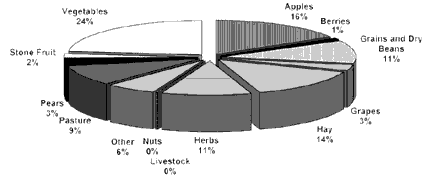 |
|
Proportion
of organic acreage by crop type, 2001.
|
These statistics indicate two very different organic farming sectors in the state. Larger scale farming in eastern Washington accounts for most of the organic acreage (82% of certified acres, 98% of transition acres) and a majority (66%) of organic growers. Average organic acreage per farm is about 92 acres in eastern Washington compared to 40 acres in western Washington. If the exempt growers were included, the numbers for western Washington would certainly increase. It will be important to understand the differing research and education needs of these two groups of organic growers.
For some crops, organic acreage is a minor factor. Organic grain production in the dryland regions of eastern Washington, for example, remains a challenge; participation there is small.
In other crops, organic acreage is now a significant portion of total production. For example, in 2001 there were about 6500 acres of certified organic apples in Washington, representing about 4% of the state’s total apple acreage. Organic pears on 1300 acres represented about 5% of total pear acreage. Washington’s organic apple acreage represents about 38% of the organic apple acreage in the United States and 21% of worldwide acreage. However, the dramatic expansion of organic apple production here and around the world is increasing competition and changing market dynamics, especially the availability of organic fruit from the Southern Hemisphere. Some varieties have experienced price declines as growth in supply exceeded the growth in demand.
Grower Perspectives
Based on recent studies from Washington State University, an organic apple orchard can produce the same yield and quality as a conventional orchard at a 10-15% higher cost. Premium prices for organic apples generally lead to higher net returns. With ongoing research, cost savings are anticipated in the near future, especially for fruit thinning. And as conventional apple production shifts to more IPM and biological control, the difference between organic and conventional will be less clear.
Growers have several motivations to try organic farming. With low prices for most farm commodities, certified organic products may offer increased returns. Organic farming can be viewed as a risk management strategy, diversifying markets, reducing the impact of loss of pesticides and other regulatory changes, and reducing liability for worker exposure or contamination. In addition, recent changes in Federal farm policy may provide funds to growers using environmental conservation practices, such as organic farming. While research results are inconclusive about the influence of organic farming on the quality of food products, growers and consumers are increasingly reporting positive experiences with organic foods.
As more growers try their hand at organic production, many of them find that certain practices that were adopted to meet the organic rules are applicable across the whole farm. I hear about this “trickle-down” effect frequently from growers and field consultants. Examples are the use of compost and increased reliance on natural biological control. Thus, as agricultural researchers address more of the specific needs of organic growers, all growers stand to benefit.
CSANR’s Role
Public agricultural institutions are responding to the increase in organic farming. The WSU Center for Sustaining Agriculture and Natural Resources (CSANR) surveyed WSU faculty about their involvement in research and education projects that were directly useful by organic growers. Over 50 faculty responded affirmatively regarding their programs, with the greatest amount of activity occurring in pest management and soil management. The results of this study, An Assessment of Organic Farming Research, Teaching, and Extension at Washington State University (CSANR Report No. 3), are available on-line at http://csanr.wsu.edu/resources/OrganicReport.pdf. A day-long symposium highlighting organic and biointensive research is being organized by the CSANR for November 8, 2002, in Yakima. This will be an excellent opportunity for growers, researchers, and ag industry representatives to learn about new developments and explore future collaboration. Details are on-line at http://csanr.wsu.edu.
The original mandate of CSANR was to support research and education on alternative practices, many of which form the foundation of organic farming. We are pursuing two funding initiatives to try to enhance WSU’s capacity to support the needs of growers interested in organic and other biointensive approaches. One initiative is a request for $510,000 for Organic Cropping Research and Education for the Northwest, submitted to the state Congressional delegation for special Federal funding similar to many other agricultural programs. Funding would help set up organic experimental land for major crops at WSU research locations, support the development of organic seed production in the region, explore organic weed control methods for annual crops, and examine the effect of production practices on food quality. These are all priority needs expressed by organic growers in the state and nationwide.
A separate proposal to the College of Agriculture and Home Economics called BIOAg (Biologically Intensive and Organic Agriculture) was chosen as one of three priorities for consideration as part of the next state funding request. The $1.8 million request over two years would support research, extension, and development of new undergraduate programs in organic and biointensive farming. The proposal would build on the expertise of existing faculty and their programs and would position WSU to better support Washington farmers in coping with the changes in the organic and conventional food sectors.
Looking Ahead
There is no doubt that organic farming will continue to expand in our state. A number of researchers have estimated that organic foods might eventually expand to 10-15% of total food sales. Price premiums will influence the expansion, as will the continued shift of conventional farming to more sustainable approaches. It is not inconceivable that for certain crops organic production could become the norm if production costs are equal to or less than other systems. An increased investment in research on organic farming would make this more likely. Central Washington is ideally positioned for organic farming of many commodities with its combination of a semi-arid climate and irrigation water. Thus, organic farming, or whatever it evolves into, will likely influence agriculture in our state for years to come and may offer Washington agriculture a measure of increased sustainability, both environmentally and economically.
For information on the proposed funding initiatives, contact David Granatstein at 509-663-8181 x. 222; granats@wsu.edu, or Chris Feise, CSANR Director, at feise@wsu.edu.
David Granatstein is a Sustainable Agriculture Specialist with WSU Center for Sustaining Agriculture and Natural Resources (CSANR) based at the Tree Fruit Research and Extension Center (TFREC) in Wenatchee. He is also a member of the Washington State Department of Agriculture’s Organic Advisory Board.
Return to Table of Contents for the July 2002 issue
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
Announcements & Upcoming ConferencesIR-4 Seeks Input for 2002 Prioritization WorkshopThe Interregional Research Project Number 4 (IR-4) was established in 1963 to increase the availability of crop protection chemistries for minor crop producers. IR-4 is a federal/state/private cooperative that aspires to obtain clearances for pest control chemistries on minor crops. Each year, dozens of new projects are undertaken by IR-4. The program receives a far greater number of requests than it can pursue, so projects are prioritized, and only the higher-priority projects are guaranteed investigation. The prioritization process takes place at an annual meeting. The IR-4 prioritization workshop for year 2002 projects will take place September 17, 18, and 19 in Orlando, Florida. Commodity representatives and growers who may be interested in helping to identify and prioritize the IR-4 projects (pesticides for residue research on specific commodities) for 2003 should fill out a Project Clearance Request form (located at the IR-4 Web site at URL http://www.cook.rutgers.edu/~ir4) and contact Washington State IR-4 Liaison Dr. Douglas B. Walsh. By providing Dr. Walsh with efficacy data and an explanation of the industry's need for a particular product, you can help him and the Western Region IR-4 program function as advocates for that product. If you have questions regarding the prioritization process, or would like to express a pesticide/commodity need for registration please contact Dr. Doug Walsh at (509) 786-2226 or dwalsh@wsu.edu. New Publication on Biotech Crops Released Despite
the exponential rise in information about crops bred using the techniques
of modern biotechnology, few reports have focused solely on the
commercialized varieties and compared their environmental impacts
with traditionally bred crops. Filling this gap, the Council for
Agricultural Science and Technology (CAST) released on June 25,
2002, a comprehensive report that critically analyzed the published
literature (including journals, reports, and regulatory documents)
on biotechnology soybean, corn, and cotton crops. Nine environmental
impact areas were studied: Despite
the exponential rise in information about crops bred using the techniques
of modern biotechnology, few reports have focused solely on the
commercialized varieties and compared their environmental impacts
with traditionally bred crops. Filling this gap, the Council for
Agricultural Science and Technology (CAST) released on June 25,
2002, a comprehensive report that critically analyzed the published
literature (including journals, reports, and regulatory documents)
on biotechnology soybean, corn, and cotton crops. Nine environmental
impact areas were studied:
In
addition, the report also includes an analysis of the published
economic impacts literature. The authors of the report are Janet Carpenter (National Center for Food and Agricultural Policy), Allan Felsot (Washington State University), Timothy Goode (Clemson University, Michael Hammig (Clemson University), David Onstad (University of Illinois), and Sujatha Sankula (National Center for Food and Agricultural Policy). Copies of the report can be downloaded as PDF files from the CAST Web site (http://www.cast-science.org). New Sustainable Agriculture and Organics Publications AvailableThe
Center for Sustaining Agriculture and Natural Resources (CSANR)
is happy to announce two new publications. Sustainable
Agriculture in Washington State
|



















