





      |
|

|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
 |
|
|
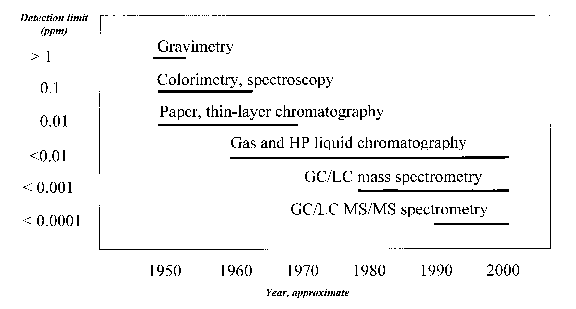
Although gravimetric methods were sufficient for tolerance enforcement of the old mainstay inorganic arsenical pesticides, the newer-generation chlorinated organics required a different approach. Colorimetry and spectroscopy methods offered greater precision. Wet-chemistry-based workups were developed that altered the spectroscopic properties of substances such as DDT and made them suitable for colormetric determinations. These methods offered some advantages, but were tedious and still imprecise.
Soon, chromatographic methods made inroads into resolving separate components from a mixed solution. Chromatography is a physical method of separation that relies on the interaction of substances within a mixture when they are exposed to both a stationary and a mobile phase. For example, remember the childhood experiment of placing a freshly cut celery stalk in a well of India ink? The ink pigments separate into their discrete colors as the ink migrates by (mobile) capillary forces up the (stationary) celery stalk. This illustrates chromatography-the elements in the mixture separate from each other as a result of repeated sorption/desorption acts during the movement along the stationary bed. Early thin-layer chromatography (TLC) and paper chromatography (PC) techniques used in the '50s and '60s separated compounds that were detected by measuring their intensity using ultraviolet and visible spectroscopy techniques.
|
Gas and high performance liquid chromatography (GC and HPLC) were developed in the 1960s but came into their own in the 1970s, becoming the methods of choice for pesticide residue analysis and replacing TLC and PC. GC and HPLC techniques efficiently "resolve" individual components from a complex mixture and can precisely quantitate how much of an individual substance is present in the mixed component sample. The primary difference between GC and HPLC is that the former relies on resolution of substances being swept through a chromatography column in the gas phase at elevated temperatures while the latter relies on the substance in solution being chromatographically separated when in contact with a solid stationary phase.
Gas Chromatography
GC is most applicable to pesticides of relatively high thermal stability and low polarity. These pesticides are easily extracted from their crop, soil, or water matrix with an organic solvent. In many cases, GC analysis can be performed on a polar analyte (often an oxidative breakdown product of the parent pesticide), providing the analyte can be chemically altered and made more volatile. A number of detectors are highly sensitive and selective for pesticides containing halogens (i.e., chlorine and fluorine), nitrogen, and/or phosphorus; these instruments can yield detections approaching 0.01 ppm (Figure 1). GC remains the method of choice for routine analysis of most chlorinated organic, organophosphorus, carbamate, and pyrethroid insecticides.
Mass spectroscopy (MS) developments in the mid-1980s dramatically enhanced the scope of detection to include most semi- to non-polar, thermally stable pesticides in use at that time. The first generation combined GC-mass spectrometers relied on Electron Impact Ionization (EI) to fragment the pesticide molecule into an array of positive mass ions. Like pieces of a puzzle, the mass ion fragment information could then be deciphered to establish the identity and quantity of the pesticide residue. GC/MS detection for many pesticide residues in crops were found to be lower than 0.001 ppm.
High Performance Liquid Chromatography
HPLC offered an alternative to GC in that it was applicable to practically any organic substance solute regardless of its volatile properties or thermal stability. It also had greater separation power than GC. Its major disadvantage during its early development was less detector sensitivity than GC. However, for certain analytes that could fluoresce or be chemically modified to fluoresce under UV illumination, HPLC sensitivity could approach or exceed that of GC.
|
Continued refinement in GC/MS and the maturation of HPLC-mass spectrometry have resulted in increasingly sensitive instruments and residue detections at even lower levels. Today's GC/MS instruments have five to ten times greater EI sensitivity than their predecessors. Bench-top laboratory instruments now come with softer ionization interfaces that increase instrument sensitivity an additional 2X to 4X, lowering the limit of detection for many pesticide residues in crops to below 0.0001 ppm. Advances in MS for HPLC, particularly improvements in the Atmospheric Pressure Ionization (API) mass spectrometers have been astounding in the last five years, revolutionizing pesticide residue analysis. Overall, the advances in instrumentation and technology have provided the analytical chemist with very powerful tools to rapidly and precisely measure extremely low levels of highly polar pesticide residues and their breakdown products. Today's technology allows analysis of virtually any matrix without the need for multi-step cleanup and chemical modification. These features are also very attractive in multi-residue tolerance enforcement procedures.
|
Over a very short period of time, we have come a long way: from gravimetric, wet-chemistry methods to highly sophisticated chromatography and spectrometry; from residue measurements in parts per thousand to fractions of parts per million. Unfortunately, our capability to use science for making sound regulatory decisions has lagged behind. While both the regulatory and science communities will agree there is probably little scientific basis for setting "zero" standards for pesticide residues in foods, public perceptions will continue to exert the greatest influence on legislation.
Dr. Vincent Hebert is an Analytical
Chemist with WSU's Food and Environmental Quality Laboratory.
He can be reached at (509) 372-7393 or vhebert
@tricity.wsu.edu.

I sat riveted to my TV in late November watching the latest phase of the Presidential election. The "trial" of the contest phase, which followed the second official certification of the Florida vote count, was being televised and the witnesses for the plaintiff were being coddled by their lawyer and excoriated by the lawyer for the defense. Of course, courteousness and civility reigned. And then it hit me. The discourse wasn't really about election interruptus, it was about our failure to understand the uncertainties associated with measurement and mistaking correlation for causation. When I viewed the whole mess in terms of science (i.e., what principles do we use to create and test hypotheses?) I wasn't going to be dragged from my TV set without kicking and screaming.
The imprecise and inaccurate nature of casting and counting votes, hidden from our view until now, struck me as parallel to the way we go about trying to decide whether the plethora of modern society's by-products are adversely affecting our health. As Dr. Hebert points out in his article on the evolution of analysis methodology, above, our ability to measure contaminant residues is greater than ever. But residue measures are virtual realities (14). Why virtual? Because every time we sample soil, water, plants, or organisms and measure pesticide X, we come up with a different number. Stated differently, there is a distribution of possible residue numbers. Measurement, by its very nature, is imprecise. The "true residue" can only be estimated, always with some degree of uncertainty.
While generating numbers has become increasingly easy, assigning meaning to residue data is more difficult than ever.. Toxicologists used to have an easy life. They could always tell when exposure to a hazardous chemical was too much-it would kill an animal or at least do some notable damage. The endpoints were obvious. Fifty years ago, we didn't see things at parts per billion or parts per trillion levels. Now that we are measuring some contaminants at parts per quadrillion levels, we know we are exposed to literally everything. Yet life seems to merrily go on. Much of today's "virtual reality" for the toxicologist involves chronic exposures, minuscule residues, and no discernible effects within a reasonable timeframe.
What about all those scary headlines asserting links between cancer and chemical X, Y, and Z? The problem with those headlines is that they are based on environmental epidemiological studies, all of which attempt to correlate exposure with some adverse outcome. But environmental epidemiological studies don't usually measure exposure. This is particularly true for pesticide studies, where next of kin are often interviewed to get an idea about what the "man" of the house was using before he succumbed. Closer examination of many epidemiological studies shows that the statistical significance of the headline associations between "exposure" and effect may be highly exaggerated or nonexistent (10).
Even studies using chemical contaminants in tissues like blood or fat as surrogates for exposure have failed to show definitive relationships between residues and effects. For example, a 1993 study linking breast cancer and DDE (the major metabolite of DDT) levels in women's blood (32) received national publicity that was followed by hearings in Congress. Although it has been recognized that breast cancer incidence among women rose in the 1980s and early 1990s, several studies subsequent to the 1993 study disputed links between DDE residues and breast cancer incidence (20). The concern over breast cancer is warranted, but our ability to figure out what role exogenous factors like low levels of environmental contaminants play in disease rates among the general population is practically nonexistent. In other words, it's easier to produce a residue number than it is to say what that number means.
Until fairly recently, public concern with environmental contaminants seemed to center on cancer. But an evolving understanding of the mechanism of carcinogenicity and a consensus that high-dose rat feeding studies are not predictive of low-level environmental exposures has tended to downplay the relationship between environmental contaminants and cancer incidence (1). A National Academy of Sciences 1996 report (22) gave strength to the idea that contaminants are not problematic for the general population with regard to cancer causation. And the good news during the 1990s continued with reports from the National Cancer Institute that incidence of many cancers was declining and the rates of increase of breast cancer were slowing to a standstill (30, 31).
But peace and quiet on the cancer front gave way in 1996 to Our Stolen Future. This highly publicized book by principal author Theo Colborn asked the question in its subtitle "Are we threatening our fertility, intelligence, and survival?" Forget cancer. Forget knocking off a few fish with insecticide runoff. Synthetic chemicals are striking at the very heart of life on earth. With a forward by Vice President Al Gore proclaiming the book to be the sequel to Rachel Carson's Silent Spring, one just assumes it must be a publication of great integrity.
Disagreement with Our Stolen Future's premise abounds among more skeptical scientists, especially those representing sectors of the economy impugned by the book. Individuals can argue back and forth all day long about the validity of the book's claims, but I believe its true relevance lies in what it represents. Our Stolen Future stands as a bridge to the 21st century for environmental advocates who were wondering what to do with their time now that the cancer scare associated with environmental levels of residues seems to have petered out. Our Stolen Future presents a comprehensive hypothesis linking just about every adverse effect under the sun to environmental contaminants affecting the endocrine system.
Linking chemical toxicity to effects on the endocrine system is an attention-getting strategy. After all, the endocrine system is linked with the nervous and immune system and has a controlling influence on reproduction, development, growth, and everyday physiology. Given that all the systems communicate with one another in feedback loops, just like a computer network, an adverse effect anywhere in the system can muck up the whole works. In essence, just about any adverse effect noted by a chemical could be interpreted as one of direct or indirect endocrine system disruption.
As the 1990s came to a close, Our Stolen Future succeeded in shifting the paradigm of "high doses cause discernible effects" (based on studies of rodents in laboratories) to "environmental exposures cause subtle effects" (on reproduction, the immune system, and behavioreffects that are not noticed until long after an exposure has taken place). All of a sudden, disparate hypotheses to explain endocrine-system-related cancers of the breast and prostate and adverse effects on reproductive systems came together under one roof. Exposure no longer needed to be at the levels associated with laboratory studies. Fetal exposure became a focal point, very much in keeping with the mandate of the Food Quality Protection Act of 1996 to manage pesticide risks for the protection of infants and children.
It's not every day that the public is treated to a shift in toxicological paradigm. Thirty years ago we were dealing with pesticide residues like DDT and industrial chemicals like PCBs that accumulate in body fat because they are metabolized and eliminated from the body very slowly (7). We weren't sure if they could build up to hazardous levels, although we knew that dead birds had very high levels of DDE in their brains (8). DDE being a neurotoxin, it was reasonable to assume that high levels would not be good for birds. Invoking the cancer scare, EPA banned DDT officially in 1973, and by 1979 PCBs fell from grace. The emphasis was still on high levels-buildups sufficient to cause an adverse effect. But the toxicological paradigm shift began a few years earlier with hypotheses that DDE did not have to be at "high" levels to affect bird populations. Instead, DDE levels commonly occurring in the environment were associated with avian reproductive failure by causing eggshell thinning (24).
When our national symbol, the bald eagle, begins to experience a population decline, people pay attention. The eggshell-thinning hypothesis with links to accumulation of DDE was a smoking gun. During the 1970s and early 1980s numerous papers reported DDE levels in bird eggshells and correlations with the thickness of those shells (8). Using correlative statistics (which show association, not causation), scientists hypothesized that populations of several predatory and fish-eating birds were declining as thinner-shelled eggs failed to hatch successfully (9, 19).
Some scientists remained skeptical of DDT's effects on declining bird populations (as opposed to individuals), and a few questioned the premise of the relationship between DDE levels and eggshell thinning (17, 28). Some laboratory studies corroborated this association and some did not. The experiments did not clearly show that hatchability was sufficiently affected to cause the reported population declines. Neither was it ever made clear to the public that the standard for determining the amount of eggshell thinning was based on comparison to museum specimens collected from different parts of the world before DDT was commercialized (2, 19). One particular observation has always vexed me, however. Not too long after DDT's demise, reports of increasing populations of various birds began to appear (3). Knowing that DDE lasts "forever," I wondered how all of a sudden bird populations with DDE still in their eggs were now making such a quick comeback.
Following the effects of Carson's Silent Spring in the 1960s and throughout the publicity of eggshell thinning in the 1970s, DDT became a symbol of everything bad about pesticides. After the banning of DDT and the continued rise in the use of the more acutely toxic organophosphate insecticides, the number of eggshell thinning studies began to wane. Although DDT was banned, scientists over the last thirty years never stopped studying it. But the 1990s brought a new respectability to DDT studies. Now the hunt was on to link DDT and recalcitrant chlorinated pesticides to adverse effects on the endocrine system.
The list of adverse effects associated with endocrine disruption has grown. So has the list of chemicals that react positively in the test-tube-type procedures used for testing this phenomenon. Sex makes good headlines, so stories of worldwide declining sperm counts have become a mainstay over the last decade. Never mind that no real decline in sperm count has ever been proven. Like pesticide epidemiological analyses, conclusions of sperm count declines are based on correlational analysis. But there is a catch-disparate sperm count studies over numerous years have been combined by meta-analysis as if they were a single data set (13, 27).
One problem with meta-analysis is the variable standards that different observers use to measure sperm counts. A second problem is the statistical model one applies to the data (11, 18). Depending on your perspective, you will either see a linear decline in sperm counts from the late 1930s to the present (16, 27) or a slight increase over the last decade and a half (23). Of course, such differences in opinion have not prevented alarming conclusions linking sperm count declines to industrial and agricultural pollution (21, 25).
Part of the toxicological paradigm shift wrought by Our Stolen Future has been a questioning of the shape of the dose-response relationship. In 1997, one researcher in particular hypothesized that for chemicals reactive with the estrogen receptor (a key endocrine system component) low doses could cause adverse effects not seen at high doses, absent systemic toxicity (29). For example, feeding rats with low doses of the drug diethylstilbesterol (DES) caused enlarged prostate glands, an effect that did not occur at higher doses. In other words, the shape of the dose-response curve was inverted. DES was an anti-miscarriage drug given to pregnant in women during the late 1950s and 1960s. It garnered a notorious reputation in the 1970s when it was discovered to have severe side effects, including reproductive-tract cancers and low fertility in offspring. DES is one of the few existing chemicals proven to have potency equal to the natural estrogen hormone. Many of the researchers jumping on the endocrine-disrupter hypothesis in the 1990s had been studying DES during the 1970s.
While low-dose DES studies garnered public attention and set off alarm bells, little has been said about studies showing effects opposite to the so-called inverted dose-response (5). With little fanfare, a study was published in 1999 completely refuting the results of the DES study (12). Furthermore, the 1999 study showed that another controversial chemical, bis-phenol A, also exhibited the standard old-paradigm relationship: any effects on the prostate gland are directly related to dose. Exposures to bis-phenol A are probably ubiquitous as the chemical leaches at very low levels from certain plastics and the polymer linings of tin cans.
Another controversial issue regarding endocrine disrupters has been the effects of exposure to multiple chemicals. One prominent EPA policy maker exuded fear in response to a 1996 Tulane University study showing that combinations of pesticides synergistically activated the estrogen receptor (4). In other words, the individual pesticides were of low potency, but when mixed together they had very prominent effects. When Tulane researchers withdrew their study in 1997 on the grounds that it was unreplicable, the action drew little notice. Since that time, numerous researchers have shown combinations of chemicals reacting with the estrogen receptor act simply in an additive manner, not a synergistic one (15, 26).
The bridge to the 21st century is paved with gold for environmental advocacy groups as the endocrine disrupter paradigms have taken hold of risk management. Witness the 1996 requirements imposed by Congress in the Food Quality Protection Act and the Safe Drinking Water Act for testing to determine whether any chemicals affect the endocrine system. But the requirement for testing will always be obscured by the interpretation of a positive result. One problem with the available testing systems is that a plethora of natural chemicals (including food biochemicals) known as phytoestrogens also test positive. Another problem is that the test-tube-type tests are sensitive over a 10-million-fold range in chemical concentrations. Thus, we're back to the high-dose testing strategy used for cancer-pump up the concentration until you get an effect. That's a nice strategy for figuring out possible effects of a chemical, but it will not give us useful answers regarding risk of an adverse effect until we can quantify real-world exposure.
As we lunge into the new millennium, we will continue to be bombarded by worries over synthetic chemicals. Such worries arise against a background of unprecedented human longevity and declines in many cancer rates. Worldwide fertility seems not to have suffered from endocrine disrupters. If it had, why would world population still be rising faster than we can accommodate it with current food production systems? Intelligence test scores in many countries have actually risen, not declined as Our Stolen Future would have us believe (6).
Yet despite the good news, we live in a world of uncertainty. We can measure synthetic chemical residues everywhere in the environment. As society demands that we lower our detection levels to see even smaller quantities, our measurements start losing their precision and accuracy. More importantly, what such low levels mean depends on who is doing the interpreting.
I guess the historic election impasse of the year 2000 has taught me a lot about toxicology. Pesticide residues are like pregnant chads. There seems to be a lot of them, but everyone is confused about what they really mean. Welcome to the 21st century!
Dr. Allan Felsot is an Environmental Toxicologist with the Food and Environmental Quality Laboratory at Washington State University's Tri-Cities campus. He can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
December 5, 2000, the U.S. Environmental Protection Agency (EPA) announced an agreement with manufacturers to phase out most uses of the agrichemical diazinon. The phase-out will begin March 2001 for indoor uses and December 2003 for lawn, garden, and turf uses.
Specifically, the phase-out works as follows:
Until the dates listed, it is still legal to purchase and use diazinon products according to label directions. Those wishing to dispose of diazinon should contact their local solid waste disposal service or their state's pesticide disposal program. In Washington, contact the Washington State Department of Agriculture's Waste Pesticides at (877) 301-4555 or wastepesticide@agr.wa.gov.
Diazinon is one of the most widely used home and garden pesticides in the United States. It tops the list of lawn chemicals used by homeowners, and is an extremely popular agent for grub and insect pest control in gardens. It is also registered for certain agricultural uses.
Eliminating most diazinon uses, according to EPA Administrator Carol Browner, will significantly reduce "the vast majority of organophosphate insecticide products in and around the home(and) help encourage consumers to move to safer pest control practice." The action is consistent with the Clinton-Gore administration's aggressive targeting of organophosphate pesticides, a class believed to pose the greatest risk to human health (especially children's health) and the environment.
The agreement reached December 5 between EPA and diazinon manufacturers Syngenta and Makhteshim Agan will eliminate seventy-five percent of diazinon use, or over eleven million pounds of diazinon annually.
For more information on this and other
EPA actions, see their website at
http://www.epa.gov/pesticides/.
Specific diazinon information can be found at http://www.epa.gov/pesticides/op/diazinon.htm.
Designed to provide the broadest, most comprehensive information about precision farming in the western United States, the Western Precision Agriculture Conference is being held January 29 through 31 in the Tri-Cities. Sponsored by the Washington State University Center for Precision Agriculture Systems and Washington State University Conferences and Professional Programs, the conference is designed to help attendees learn how to maximize profitability by matching crop production practices and inputs to the needs of their unique field areas.
New to this year's conference is a hands-on precision agriculture (GIS, GPS) workshop to be held at the Consolidated Information Center on the WSU Tri-Cities campus. Space is limited, so register early. All other sessions and exhibits will be at the Pasco DoubleTree Hotel (800-222-TREE or 509-547-0701). Registration is $189 before January 10, and $229 thereafter. The hands-on precision ag workshop is $40 per session, or $120 for all three sessions (see website or request a brochure for details). Continuing Education Units and Certified Crop Advisor credits are available.

The Food and Environmental Quality Laboratory (FEQL) Advisory Board met for the fourth time at the Washington State University (WSU) Tri-Cities campus on November 21, 2000. I opened the meeting, then invited WSU administrators to address the group. Washington State University (WSU) College of Agriculture and Home Economics Dean James Zuiches outlined the university's current budgetary situation. Department Chair of Entomology, John Brown reminded board members of suggestions they had made for the FEQL faculty members at their last meeting (see "FEQL Advisory Board Prepares for 2000," AENews Issue No. 165, Jan. 2000).
Next, we addressed board structure and policies, including terms of office. Marilyn Perkins and I will retain our current Vice Chair and Chair positions through June 30, 2001. In response to an Advisory Board request for more communication from FEQL members, Dr. Allan Felsot volunteered to coordinate a quarterly e-mail update.
Dr. Catherine Daniels briefed the group on a proposal she had submitted for regional funding to support a state Pest Management Center (PMC) within the existing Pesticide Information Center of the FEQL. The board agreed to serve as a stakeholder advisory committee for the new PMC. The Advisory Board reviewed several crop profiles generated through the Pesticide Information Center (PIC) and complimented the effort.
Dr. Vincent Hebert introduced himself to the Advisory Board with an overview of his activities since joining the FEQL in July 2000. He emphasized the need to have the FEQL certified as a Good Laboratory Practices (GLP) facility and praised Doria Monter-Rogers for her willingness to serve as the on-site Quality Assurance Officer. Dr. Hebert briefed the group on several collaborative projects that he has initiated. He expressed the need for a liquid chromatograph linked to two successive mass spectrometers in order to succeed in analysis of newer more hydrophilic pesticides.
Dr. Douglas Walsh shared information he had collected on both beneficial and pestiferous insects found in riparian buffer zones. This was an area of research the Advisory Board had suggested FEQL personnel pursue. Dr. Walsh, the Washington State Liaison Representative for the nationwide Interregional Research Project #4 (IR-4), talked about IR-4 projects scheduled for completion by FEQL members this coming year.
Dr. Allan Felsot referred the Advisory Board to his articles published in the Agrichemical and Environmental News on buffer zones and on genetically modified organisms (GMOs). Dr. Felsot and WSU Tri-Cities Dean Larry James proposed that Dr. Felsot become more involved with undergraduate education on campus. Dr. Felsot stated his interest in working with the Columbia Basin College faculty to coordinate a General Agriculture degree through which students could emphasize one of several specific disciplines. His effort toward teaching would be rewarded by additional graduate research assistantships from the Agricultural Research Center, thereby allowing him to continue his toxicology research.
Sally O'Neal Coates presented an update on success of the FEQL's primary communication tool, the monthly Agrichemical and Environmental News newsletter.
Looking ahead toward other issues to be addressed by the Advisory Board and by FEQL, farm worker safety (specifically re-entry intervals, or REIs) and air quality (specifically as relating to the Americans with Disabilities Act) were identified as possible issues to explore. FEQL board and/or faculty members will either address these issues at future meetings or invite experts to speak to these topics at the spring meeting of the Advisory Board, which was scheduled for Tuesday, April 17, 2001.
Scott McKinnie is Executive Director of Far West Agribusiness Association (http://www.fwaa.org/) and Chair of the FEQL Advisory Board. He can be reached at (509) 464-4886 or at scott@fwaa.org.
Beginning January 1, 2001, the state of Oregon will no longer accept new public or commercial pesticide applicator or pesticide consultant licenses from Washington or Idaho as reciprocal license equivalents. There will be no change in reciprocity for private applicator licenses.
The Oregon Department of Agriculture (ODA) announced earlier this year that reciprocity with Idaho and Washington had become increasingly difficult to maintain due to the differences in the three states' certification and licensing programs, as well as differences in certification period, license duration, and license categories. Only the private applicator program remains relatively consistent between states.
In an effort to minimize the impact of this change for current license holders, ODA will be issuing fully certified licenses for all persons applying for a renewal of a reciprocal license.
Those individuals with established reciprocal licenses will follow these steps to renew their reciprocal license:
Any person wanting to be licensed for the first time in Oregon must take, and pass, the Oregon certification examinations for the license type desired.
For more information, refer to the ODA website at http://www.oda.state.or.us/pesticide/info.html, or contact Janet Fults at (503) 986-4635. A list of frequently asked questions can be viewed at http://www.oda.state.or.us/pesticide/reciprocal.html.
Cluster fly is widely distributed throughout the United States. The fly enters structures in the fall seeking areas in which to overwinter. They often collect in attics, basements, wall voids, closets, or any dark, protected area. There, they gather in groups or clusters and enter a dormant state to wait out the cold weather months.

Slightly larger than houseflies, cluster flies are a nonmetallic dark gray. They have no markings on the thorax and the thorax has a hairy appearance. The abdomen is dark gray with irregular lighter patches.
Cluster flies lay their eggs in soil. When the larvae hatch, they are parasitic specifically on earthworms, making eradication difficult (see below). From late spring through early fall, the flies will produce about four generations.
Cluster flies do no damage per se, but can be a great annoyance when they become active in the spring. They can also be a problem on warmer days during the winter, when numbers of flies may break dormancy prematurely. As temperatures warm, the flies may emerge off and on for several weeks. They tend to congregate on windows in the sunniest rooms of the structure, and are often sluggish. While cluster flies are very adept at finding their way into a structure, they are not as capable when seeking to exit. This can sometimes result in hundreds of flies congregating in various rooms.
Most flies of this size breed in garbage, carcasses, or other rotting organic matter. When breeding sites are eliminated (cleaned up), populations of these flies are quickly reduced. Not so with cluster flies. Due to the subterranean dietary preferences of the larvae, cluster flies are not eradicated through sanitation.
When cluster flies emerge in the spring, they will readily exit the structure if they can find an open, screenless window. If they congregate near a window that won't open, it is difficult to get them to move to another site. If you cannot get them to an open window, you can vacuum them up with a hose attachment on your vacuum cleaner. Remove the vacuum bag and discard it in an outside trash container afterward. If a small number of flies are present, insect glue traps can be taped in the corners of the affected windows and discarded after the insects are caught.
To prevent a cluster fly problem, try to eliminate their entrance paths. Check the structure's exterior, concentrating on the side of the house that gets the most sun. Look for and replace missing vent screens. Windows and doors that do not shut completely can be made more secure with weather stripping. Seal cracks along soffits, eaves, and siding, and secure loose boards. Such inspection and exclusion work can be time-consuming, but is generally inexpensive. And it beats "herding flies" after a big indoor emergence!
Jack Marlowe is the owner of Eden Advanced Pest Technologies (http://edenpest.com) and current President of Washington State Pest Control Association. He can be reached at edenapt@olywa.net or (800) 401-9935.
Washington State University offers PRE-LICENSE courses (for those who do not have a license and need one) and RECERTIFICATION courses (for those who need to renew their current licenses). Fees are $35 per day if postmarked 14 days before the program, otherwise $50 per day. This fee DOES NOT include WSDA license test fee, which ranges from $25 to $170; for information on testing and fees, contact WSDA at (360) 902-2020 or http://www.wa.gov/agr/test/pmd/licensing/index.htm. Recertification courses offer 6 credits per day.
In reviewing the November 2000 postings in the Federal Register, we found the following items that may be of interest to the readers of Agrichemical and Environmental News.
In the November 17 Federal Register, EPA announced that companies that hold the pesticide registrations for chlorpyrifos enduse pesticide products have asked EPA to cancel or amend their registrations. These requests for voluntary cancellation and amendment are the result of a memorandum of agreement signed by EPA and the basic manufacturers of chlorpyrifos on June 7, 2000. These cancellations and requests for amendment are parallel to those announced in the September 20 Federal Register except that the first covered requests from basic chlorpyrifos manufacturers while this notification covers requests by the registrants who are customers of these basic chlorpyrifos manufacturers. (Page 69518) (For a more detailed discussion of this action see PNN notification 2000-283 on the PNN web page www.pnn.wsu.edu.)
In the November 22 Federal Register EPA announced that the draft document "Guidance for Pesticide Registrants on Bee Precautionary Labeling" is now available. The document is intended to provide guidance to registrants and others regarding EPA's policy for bee labeling statements for pesticide products that are toxic to bees. This document is available electronically at the following URL: http://www.epa.gov/pesticides/. Look under Open Comment Periods: Draft PR Notices. (Page 70350)
In the November 22 Federal Register, EPA announced that it was seeking comment on a draft Pesticide Registration (PR) Notice titled "Elimination of Phenol Resistance Testing for Antimicrobial Disinfectant and Sanitizer Pesticides.'' This draft notice provides guidance to registrants concerning the discontinuation of phenol resistance testing as a part of efficacy testing for antimicrobial disinfectants and sanitizers. An electronic copy of this draft PR notice is available on EPA's web page at: http://www.epa.gov/pesticides/. (Page 70352)
The PNN is operated by WSU's Pesticide Information Center for the Washington State Commission on Pesticide Registration. The system is designed to distribute pesticide registration and label change information to groups representing Washington's pesticide users. PNN notifications are now available on our web page. To review those sent out in the month two months prior to this issue's date, either access the PNN page via the Pesticide Information Center On-Line (PICOL) Main Page on URL http://picol.cahe.wsu.edu/ or directly via URL http://www.tricity.wsu.edu/~mantone/pl-newpnn.html. We hope that this new electronic format will be useful. Please let us know what you think by submitting comments via e-mail to Jane Thomas at jmthomas@tricity.wsu.edu.
| Chemical (type) | Federal Register | Tolerance (ppm) | Commodity (raw) |
|
||
| Yes/No | New/Extension | Exp. Date | ||||
| sulfentrazone (herbicide) | 11/9/00 | 0.1 | horseradish | Yes | New | 12/31/02 |
| pg. 67272 | ||||||
| Comment: This time-limited tolerance is being established in response to EPA granting a Section 18 for the use of sulfentrazone to control broadleaf weeds in Illinois horseradish. | ||||||
| pyriproxyfen (insecticide) | 11/15/00 | 0.1 | stone fruit | Yes | Extension | 12/31/02 |
| pg. 68912 | ||||||
| Comment: With this action EPA is re-establishing this tolerance. This is in response to EPA again granting a Section 18 emergency exemption for the use of pyriproxyfen to control San Jose Scale in California stone fruit. | ||||||
| fenhexamid (fungicide) | 11/21/00 | 15 | pear | Yes | New | 12/31/02 |
| pg. 69876 | ||||||
| Comment: This time-limited tolerance is being issued in response to EPA granting a Section 18 for the use of fenhexamid for post-harvest use to control Botrytis on California pears. | ||||||
W.S.U. Pullman Home Page Comments and questions: cdaniels@tricity.wsu.edu Technical Assistance: scoates@tricity.wsu.edu Copyright © Washington State University / Disclaimer Electronic Publishing and Appropriate Use Policy University Information: 509/335-3564