
February 2003, Issue No. 202
A monthly report on environmental
and pesticide related issues
Open Forum: In an attempt to promote free and open discussion of issues, Agrichemical and Environmental News encourages letters and articles with differing views. To discuss submission of an article, please contact Dr. Allan Felsot at (509) 372-7365 or afelsot@tricity.wsu.edu; Dr. Catherine Daniels at (509) 372-7495 or cdaniels@tricity.wsu.edu; Dr. Doug Walsh at (509) 786-2226 or dwalsh@tricity.wsu.edu; Dr. Vincent Hebert at (509) 372-7393 or vhebert@tricity.wsu.edu; or AENews editor Sally O'Neal Coates at (509) 372-7378 or scoates@tricity.wsu.edu. EDITORIAL POLICY, GUIDELINES FOR SUBMISSION.
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Weed Control in Container Crops
Sanitation and Cultural Practices Are Key
Dr. James Altland, Nursery Crops Extension, OSU
Container production of nursery crops creates a unique environment that presents challenges for weed control. Different from other fruit, vegetable, or seed crops, ornamental plants are grown for their visual appeal, not food or fiber. So not only do weeds in container crops compete for nutrients, water, and light, they also decrease the crop’s market value because they are unsightly. Weeds also harbor insects, disease organisms, and vertebrate pests. Marketable nursery crops must be virtually weed free.
Effective weed management in nursery crops involves a combination of sound sanitary and cultural practices along with proper use of preemergence herbicides. Sanitation practices will reduce the number of weed seeds in a container nursery, while preemergence herbicides are used to prevent weeds from growing. This article will discuss how sanitation and preemergence herbicides work synergistically to improve weed control.
Sanitation
Weed control in container production must include preventative measures. In field production where crops are planted directly in soil, weeds can be efficiently controlled after they germinate with directed sprays of herbicides or mechanical cultivation. However, in container crops where directed sprays are not feasible, there are few options other than weeding by hand (a costly remedy). Thus, a successful container weed management program should prevent weed germination.
The first step to effective weed management is sanitation. A common characteristic of weeds in containers is their ability to produce prolific numbers of small seeds. Practices that minimize the number of weed seeds in the production system will improve weed control. The more weed seed allowed to contaminate containers, the higher the probability weeds will germinate in areas where the herbicide has been weakened (discussed under “Herbicide Use,” below).
The following are some sanitation practices that should be considered for reducing weed seed numbers in containers.
Weed Control Under Containers
Containers should be placed on covered ground. The surface cover can be gravel, plastic, or woven weed fabric. Weeds under the covered area are usually suppressed, but weed seeds may germinate in debris on top of the surface cover, their roots penetrating down through the cover.
Weed control and sanitation under and around the containers is almost as important as weed control within the containers. Weeds growing between containers are an immediate source of weed seeds. Bittercress (Cardamine hirsuta) and oxalis (Oxalis corniculata) growing in debris spilled on weed fabric propel seeds several feet, rapidly infesting nearby containers. Other weeds like eclipta (Eclipta alba) establish in drainage holes and compete for water and nutrients.
FIGURE 1 |
FIGURE 2 |
 |
 |
| Weeds (bittercress shown above) can germinate in small piles of debris spilled on weed fabrics. | Eclipta often infest containers through drain holes, after which their roots aggressively compete for nutrients and water. |
Between crops when beds are empty, existing weeds should be removed or chemically controlled, fresh stone or new weed fabric should be installed if necessary, and the area should be swept to remove all debris.
Weed Control in Non-Crop Areas
Eliminating weeds in non-crop areas such as roadways, drainage ditches, and between hoop houses will drastically reduce production of weed seeds and improve weed control. Routine mowing can prevent weeds from setting seed.
FIGURE 3 |
FIGURE 4 |
 |
 |
| Weeds in roadways, aisle ways, and between greenhouses should be controlled to prevent their spread into containers. This is an easy and effective way to reduce weed pressure. | Weeds in non-crop areas, like those shown here growing next to containers, disseminate large numbers of seed, rendering herbicides in containers less effective. |
Mechanical means such
as hoeing or plowing can be used to remove weeds, though this makes the
area susceptible to soil erosion. Herbicides provide effective control.
Postemergence herbicides can be used to eliminate existing weeds, while
preemergence herbicides can be used to prevent re-infestation. Maintaining
weed-free non-crop areas is probably the easiest and most effective sanitary
practice for reducing weed seeds in containers.
Weed Control on Bark Piles
Bark piles should be kept weed-free.
Not only will weeds growing on bark piles generate seeds that can be blown
into containers, they also deposit seed and/or vegetative propagules directly
into the media that will be used for potting. When bark piles are kept
weed-free, they are generally not a source of weed seeds (Cross and Skroch,
1992).
FIGURE 5 |
 |
| Weeds growing in bark piles add seeds and vegetative propagules to the bark, resulting in greater weed numbers in containers. |
Steam pasteurization, solarization, composting, and fumigation are among the treatments that will kill seed and other propagules in bark piles. These treatments are too expensive to be used regularly in most nursery operations; maintaining a weed-free bark pile will eliminate the need for treatment.
Clean Pots
Use of clean or new pots for propagation and/or canning will also reduce the number of weeds in containers. A common characteristic of container weeds is their small seed size. Seeds cling to the side of containers along with other debris. Research has demonstrated that by simply washing propagation pots with pressurized water, the number of germinated weeds can be reduced to 1/6 the number of weeds from dirty pots (Bachman and Whitwell, 1995).Weeds germinated around the edges of dirty pots, compared to few or no weeds when new or cleaned pots were used.
Wind Breaks
Even if weeds are effectively controlled throughout your nursery, you can’t control weeds on neighboring properties. Weed seed from neighbors will likely blow into your containers. Use of a wind break such as a tall weed-free hedgerow or fence to minimize the amount of seed entering the nursery will improve weed control. The hedgerow or fence need not be permanent. A local nursery lined a side of their property (bordering a weedy lot) with large trees and shrubs in 15-gallon pots. This solution does double duty, providing additional production area while blocking weed seed from entering the nursery.
FIGURE 6 |
FIGURE 7 |
 |
 |
| Seeds of some weeds, like common groundsel shown above, have an attached white pappus (hairy filament structure) that aids in wind dispersal. These and similar weed seeds are commonly blown in from nearby properties. | A hedgerow such as this can help block weed seed from entering your nursery property. |
Start with Weed-Free Liners
Use of weed-free liners is critical, especially when dealing with weeds that have extensive root systems such as oxalis, liverwort (Marchantia polymorpha, which has rhizoids instead of roots), and pearlwort (Sagina procumbens). Because roots from these plants can generate new plants, thorough hand weeding to remove shoots and roots is necessary. A single escaped weed can generate new plantlets at an alarming rate. One bittercress plant can produce up to 5000 seeds in just five weeks (Bachman and Whitwell, 1995).
Cultural Practices
Research currently being conducted at Oregon State University (OSU) is demonstrating how fertilizer placement affects weed establishment. Growers typically apply fertilizers by topdressing (applying fertilizer to the container surface after potting), dibbling (placing fertilizer directly under the plant root ball while potting), or incorporation (mixing fertilizer with bark prior to potting). When fertilizers are dibbled, nutrients are not available at the container surface, thus weeds often fail to germinate and those that germinate grow poorly. Control of common groundsel (Senecio vulgaris), oxalis and prostrate spurge (Chamaesyce prostrata, syn. Euphorbia prostrata) is greatly improved by dibbling fertilizers compared to topdressing or incorporating. If dibbling fertilizers is not an option, incorporating is less favorable to weed growth than topdressing. Research (including ours) has demonstrated dibbling fertilizers also improves growth of most crops compared to topdressing or incorporating (Meadows and Fuller, 1984).
FIGURE 8 |
 |
| Fertilizer placement options. From left to right: topdressing, dibbling, incorporation. |
Other OSU research has evaluated
the influence of cultural practices on liverwort control (Svenson, 1998;
Svenson et al., 2001). Liverworts thrive in moist environments with high
levels of available nitrogen and phosphorus. Thus any practice that allows
the container surface to dry quickly, or removes nitrogen and phosphorus
from the container surface, results in improved liverwort control. Cultural
practices that reduce liverwort infestations include increasing air circulation,
using mulch, using coarse container media (container surface dries more
quickly), and dibbling or incorporating fertilizer instead of topdressing.
Herbicides such as Ronstar (oxadiazon) and oryzalin alone failed to provide
adequate liverwort control in OSU research. It was concluded that a combination
of herbicides and cultural practices was necessary for liverwort control.
FIGURE 9 |
 |
| Liverwort thrives on high nutrient levels, moist soil, high humidity, and shade. |
Herbicide Use
Sanitation is the first step in effective weed control; proper herbicide use is the second. Herbicides form a chemical barrier over the container surface. Though different herbicides have different modes of action, preemergence herbicides provide control at the point where a weed emerges from a germinating seed through the chemical barrier. If the chemical barrier is incomplete, there will be a gap where weed seed can successfully germinate and grow. Several common practices disrupt the chemical barrier, including poking holes in the barrier with fingers or hands while moving containers, dropping containers, and allowing containers to blow over. All such activities should be minimized to prevent disruption of the chemical barrier. This concept should be explained to each member of the work crew on a nursery site.
FIGURE 10 |
 |
| Herbicides form a chemical barrier (pink) over the surface of the potting medium. As weeds germinate within the chemical barrier, they are either killed or inhibited from growing. |
Pulling uncontrolled weeds (whether pre-existing or those that manage to emerge through the chemical barrier) will also create gaps in the chemical barrier. Weeds should be pulled before they go to seed. However, soon after removing weeds, herbicide should be applied to create a complete chemical barrier and prevent germination of more weeds.
To create an effective chemical barrier over the container surface, herbicides should always be applied at the rate specified on the label. If rates are too low, the chemical barrier may not be sufficient to prevent weed growth, and if rates are too high the herbicide may cause crop injury. Also, it is important to apply herbicides uniformly. When herbicides are not applied uniformly, weeds will emerge in areas with insufficient herbicide. Use properly calibrated equipment that is functioning correctly. Even when this is done, flaws in equipment engineering may still result in non-uniform applications. Even with trained staff using properly calibrated equipment, the actual amount of applied herbicide can vary throughout the treated area. In one study with granular herbicides, the variation ranged from 0.5 to 2.2 times the intended rate (Darden and Neal, 1999). Meticulous attention should be given to equipment calibration and application uniformity. Steps can be taken to ensure that proper herbicide rates are applied, such as using collection pans placed throughout the application area to measure uniformity and help identify regions that might have weak herbicide barriers. (This is similar in concept to using rain gauges to measure irrigation uniformity.)
Conclusion
The most effective weed management system for container production combines sanitation to reduce weed seed numbers with proper use of chemical herbicides to create a barrier that prevents growth of the few seeds that elude sanitation efforts. If herbicide use is over-emphasized while neglecting sanitation, increased numbers of weed seeds will find a place to germinate where the chemical barrier has been weakened. On the other hand, if sanitation is made a priority while herbicide applications are neglected or implemented improperly, the few weed seeds that are present will emerge and quickly generate more seeds.
James Altland is with the
North Willamette Research and Extension Center of Oregon State University.
He can be reached at james.altland@orst.edu
or (503) 678-1264, ext. 46.
REFERENCES
Bachman, G. and T. Whitwell. 1995. Hairy bittercress seed production, dispersal, and control. Proc. South. Nurs. Res. Conf. 40:288-290.
Cross, G.B. and W.A. Skroch. 1992. Quantification of weed seed contamination and weed development in container nurseries. J. Environ. Hort. 10:159-161.
Darden, J. and J.C. Neal. 1999. Granular herbicide application uniformity and efficacy in container nurseries. Proc. South. Nurs. Res. Conf. 44:427-430.
Meadows, W.A. and D.L. Fuller. 1984. Plant quality and leachate effluent as affected by rate and placement of Osmocote and SREF on container grown woody ornamentals. Proc. South. Nurs. Res. Conf. 29:75-79.
Svenson, S. 1998. Suppression of liverwort growth in containers using irrigation, mulches, fertilizers and herbicides. Proc. South. Nurs. Res. Conf. 43:396-402.
Svenson, S., J. Paxson, and K. Sanford. 2001. Composts and shading influence Marchantia infestations in container grown nursery crops. Proc. South. Nurs. Res. Conf. 46:445-447.
Go to this issues Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
The PNN
A Gem of a Program
Jane M. Thomas, Pesticide Notification Network Coordinator, WSU
Oh good! Another chance to brag about the PNN (that's “Pesticide Notification Network” for the uninitiated). What?! You haven't heard about the PNN? Well, pull up a chair.
The PNN is a great service that makes information about pesticide product registrations and label changes widely available to Washington State's agricultural community. It is funded by the Washington State Commission on Pesticide Registration (WSCPR) and is maintained by the trusty staff at the Washington State Pest Management Resource Service (WSPRS) at Washington State University (WSU). At its inception in 1995, the WSCPR was charged with developing a system for tracking the availability of effective pesticides for minor crop and emergency uses in Washington. The Commission asked WSU to take on this task and the end result is the PNN. The PNN tracks and communicates (through a clever set of mechanisms described below) Section 18 exemptions, Section 24c (SLN) registrations, new product registrations, label changes, proposed product cancellations and use deletions, and more. Brilliant? We think so. In fact, the PNN system has been described as “a gem.”
The Two Faces of the PNN
Like a gem, the PNN is multi-faceted. Well, now, that may be a tiny exaggeration. It may be more accurate to say that the PNN is bi-faceted or perhaps semi-faceted. In short, information is distributed two ways through the PNN: via a subscription network and via a Web site.
The PNN subscription network is the heart of the PNN system (the gem’s “crystalline structure,” as it were; OK, I’ll stop…). The way this works is that the PNN staff tracks information (types of information described more completely in the section following), logs it into a database, and sends the information to relevant subscribers. What makes the PNN system different from a basic computer listserve is the word “relevant.” Rather than pelt every subscriber with every piece of data we process, the PNN database sorts the information so that folks receive only the notifications that are of interest to them. When people subscribe to the PNN, they indicate both the crops and pesticide types of interest to them; only messages related to these crops and pesticide types reach that subscriber. For example, a plant pathologist working on raspberries receives notifications that have to do with the use of fungicides on raspberries (no insecticides, no herbicides), while the contact for the Washington Red Raspberry Commission receives all raspberry-related PNN notifications.
Another way the PNN subscription network differs from a computer listserve is that it accommodates a range of technological needs. Notifications are sent out via e-mail, fax, or US mail. Each subscriber chooses his/her preferred method.
PNN subscribers include commodity commission representatives, WSU extension personnel, and others involved in agriculture. Most PNN subscribers represent or communicate with a number of end-users, so they pass the information along to their constituents. Currently the PNN has 161 subscribers.
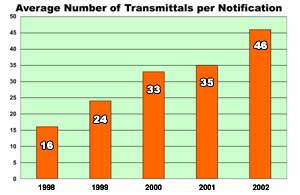
In 2002 the PNN sent out 338 different communiqués (“notifications”) via a total of 15,476 e-mails, faxes, and letters (“transmittals”). While the number of notifications was down slightly from 2001, the number of transmittals for the year was up by more than two thousand, indicating an increased distribution for each notification. In 2001 the average number of transmittals for each notification was 35; this increased to 46 in 2002. The charts below summarize PNN activity from 1998 through 2002 and indicate an overall growth in PNN distribution. (While the PNN was up and running in 1997, the database underwent a significant modification in 1997 and numbers for this year are not directly comparable to those for subsequent years.)


The second way the PNN, multi-faceted gem that it is, distributes information is via its PNN Web site (URL: http://www.pnn.wsu.edu). Useful information is posted here including Section 18 Exemptions and Special Local Needs (SLN or 24c) registrations and their associated labels. For the past two years the PNN Web site has also been home to the State Restricted Use Pesticide (SRUP) list compiled each year by WSU. New this past year is the Miscellaneous Information section of the PNN home page. Here we post pesticide-related information in electronic formats, such as PDFs (portable document format files) detailing WSDA’s clopyralid ban on turf and EPA’s response to questions about pesticide use on mixed crops. A PDF version of the PNN subscription form is also posted on the home page. Interested parties can now print out the form, complete it, and return it to WSU. In the past year over 2800 visits to the PNN Web site have been recorded.
The Content of the PNN
The PNN distributes the following specific types of information:
- Section 18 Exemptions: Notifications are sent to subscribers when new Section 18s are issued or when existing exemptions are amended. Section 18 requests are posted on the PNN Web site and Section 18 labels are also posted when the emergency exemptions are approved by EPA.
- Section 24c (SLN) Registrations: Notifications covering newly issued SLNs, as well as revisions and cancellations, are distributed via the PNN subscription network. Information on both requested and granted SLNs is also posted on the PNN Web site. When electronic copies of SLNs are available, these are posted as well.
- New Product Registrations: PNN notifications are sent when new Section 3 labels are registered in Washington and when new supplemental labels are issued.
- Label Changes: Significant label revisions (changes to crops, pests, signal words, or ingredient information) will trigger a PNN notification.
- Proposed Product Cancellations and Use Deletions: PNN notifications are sent when these actions are noticed in the Federal Register for products registered in Washington. This portion of the PNN service has been expanded to include distributing notifications about proposed use deletions or cancellations for homeowner products.
- Miscellaneous/Regulatory Issues: This may include solicitations for regulatory input and information on new regulations. Notifications are sent to subscribers describing the action or information and, if available electronically, the information is posted on the PNN Web site.
The Players of the PNN
The information distributed on the PNN comes from a variety of sources. Registration information and revised labels provided by the Washington State Department of Agriculture (WSDA) are a critical source of PNN information. Based on these the PNN provides subscribers with information on new pesticide product registrations as well as keeping them informed of significant label changes (e.g., additions or deletions of usage sites, or changes to signal words). The Federal Register is another source of information. The PNN distributes relevant information when the Environmental Protection Agency (EPA) publishes manufacturer's proposed use deletions or product cancellations in the Federal Register. Through a variety of other channels, EPA, US Department of Agriculture (USDA), WSDA, and individual registrants are all information sources for the PNN. Were it not for the efforts of all involved, especially WSPRS's own Charlee Parker who sorts the incoming registrations and revised labels from WSDA, the PNN would not be able to effectively distribute the information that it does. My thanks to all who give me input (and give me input, and give me input) for the PNN.
Rave Reviews for the PNN
I'm not the only one who thinks that the PNN is pretty great. But don’t take my word for it; we have survey data to prove it. In late 2001, PNN subscribers were queried about the PNN. From this exercise we learned, among other things, that 69% of respondents found PNN information “often” or “always” useful, that the information was received quickly enough to be useful (85% said “often” or “always”), and that the level of detail was neither too little nor too great for the majority (71%) of respondents. If you are a doubting Thomas, you can find a more complete summary of our survey results in the January 2002 issue (No. 189) of this fine publication.
The PNN Looks Ahead
Last year, because of funding cuts, the PNN budget was decreased by the WSCPR. This meant services had to be reduced and the practice of sending out PNN notifications covering things like EPA PR notices, risk assessments, and re-registration eligibility documents (REDs) was discontinued July 1. We also discontinued posting the PNN notifications on the PNN Web site. It is expected that this reduced level of service will continue in effect for some time.
Through the PNN, the WSCPR
provides a useful service to Washington's agricultural community. If you
are interested in subscribing to the PNN (did I mention it’s FREE?),
give me a call (509-372-7493) or simply fill out the subscription form
found on the PNN home page at http://www.pnn.wsu.edu
and send it along. We would be happy to have you join the ranks of the
elite PNN subscribers. While the PNN, like a gem, is “brilliant,”
“clear,” and “multi-faceted,” it’s not “hard”
to become part of it.
Jane M. Thomas is the PNN Coordinator. She also functions as the Western
Region Pest Management Center's Comment Coordinator for the Pacific Northwest,
keeping EPA informed of Northwest growers' and researchers' perspectives
on proposed regulatory actions and other matters concerning pest management.
Jane can be reached at jmthomas@tricity.wsu.edu
or (509) 372-7493.
Go to this issue's Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Waste Pesticide Disposal
WSDA Offers Important Service
Joe Hoffman, Waste Pesticide Program Coordinator, WSDA
A Brief History of Pesticides
Pesticide usage dates back almost as far as recorded history. In the biblical era, armies would salt and ash fields of the conquered to prevent crop growth. Around 1000 BC, Homer referred to sulfur used in fumigation and other forms of pest control. Pesticide use and development grew at a snail's pace for many centuries and was limited to naturally occurring materials such as arsenic, tobacco, nicotine, and sulfur until the early 1800s when the knowledge of chemistry began to grow. The next 100 years saw exponential growth of inorganic and heavy-metal based pesticides and with it growing concern about their use, including marketing scams promoting useless materials. These concerns culminated in the Federal Insecticide Act in 1910.
The development and regulation of pesticides continued to accelerate in the 1930s as organic chemistry grew and entirely new classes of pesticides were discovered. TEPP (discovered in 1938) was the first organophosphate pesticide. The insecticidal properties of DDT were described a year later, followed shortly by 2,4-D, the first truly selective herbicide. Because of concern about the widespread growth and use of pesticides, the Federal Insecticide Fungicide and Rodenticide Act (FIFRA) became law in 1947. Worries about pesticide residues on food led to the setting of tolerances on raw food and feed in 1954. Pesticide development and use continued to grow and many well-known pesticides were discovered in the late 1950s and the 1960s.
Ah, The Good Old Days |
|
 |
 |
| This container of Calcium Arsenate was manufactured in the 1930s. It was collected near Ellensburg in 1993. Some of these old labels were works of art. Safety and Use precautions have changed greatly the last seventy years. | This container of Lead Arsenate is in extremely good condition considering that it had been in a storage shed for over 70 years before being turned in at a Wenatchee area collection event. |
Public concern about the environment in the 1960s led to the creation of the Environmental Protection Agency (EPA) in 1970 during the Nixon Administration. The EPA became responsible for the registration of pesticides and began to weigh the environmental consequences of some pesticides against their economic value. They soon suspended the registration of alkylmercury compounds as seed treatments and cancelled most uses of DDT in 1973. This was followed by the cancellation of all non-termiticide uses of aldrin and dieldrin in 1975. The following year most pesticidal uses of mercury compounds were cancelled. EPA continued to cancel extremely persistent and highly toxic pesticides. This began to cause another problem.
What to Do with Waste Pesticides?
Many people did not know what to do with the now worthless stocks of cancelled pesticides they possessed. Some decided to use them anyway, while some others followed the old label language, which instructed them to bury excess pesticides and their wastes in trenches located away from water supplies. A few counties across the United States in which agriculture was predominant established pesticide dumps, most of which were not controlled. This led to undesirable situations and eventual closure of the sites. However, the majority of people quit using the cancelled products and left them stored in barns, sheds, garages, trailers, and other locations.
In 1980, North Carolina was the first state to take an active role in assisting farmers with disposal of obsolete stocks. They applied for and were granted an exemption from federal transportation laws to allow their pesticide investigators to transport unwanted pesticide products found on farms to several warehouses within the state. Once a sufficient amount of pesticides was accumulated they would hire a hazardous waste company to transport them to an appropriate landfill. This was the beginning of the first state-sponsored waste pesticide disposal program. The North Carolina program was responsible for disposing of nearly forty percent of the waste pesticides disposed by government-sponsored programs in the United States during the 1980s.
What NOT To Do with Waste Pesticides |
|
 |
 |
| At a Washington State collection event, one customer brought in a Jim Beam Whiskey bottle containing Parathion. The instructions handwritten on the label advise mixing two tablespoons of the concentrate to three gallons of diluent. What diluent? Didn't say. | This Quaker Oats container simply had "Vapotone" handwritten on it. Vapotone is DDT 50% Wettable Powder. The "50 % DDT WP 3.6#" was written on the container by WSDA staff to identify its contents and weight before turning it over to the hazardous waste contractor. |
Disposal Programs Emerge and Evolve
Word of North Carolina's program began to spread, but stiff regulatory hurdles, liability concerns, and high prices for legal disposal stymied the adoption of the program in other states. Throughout the 1980s, EPA continued to cancel pesticides such as the highly toxic and persistent endrin and later, the widely used contact herbicide dinoseb. During this time, a small but increasing amount of full and partial pesticide containers were beginning to be abandoned on public lands. It was suggested that farmers should hire hazardous waste firms to dispose of their excess pesticides. However, very few did this due to a requirement that they must obtain an EPA hazardous waste site identification number for their farm and comply with complicated hazardous waste disposal and reporting laws. This was in addition to the prohibitive cost of disposal and fear of unknown long-term liability issues. At this point, few people wished to admit they possessed a "banned" pesticide.
Here in Washington State, a farm survey commissioned by the Department of Ecology in 1987 showed there were more than 86,000 pounds of unusable pesticides wasting away on Washington farms. Something creative needed to happen to begin resolving this problem.
Washington State Gets Busy
Many people in Washington State became involved in the idea of dealing with waste pesticides after the 1987 survey. Most notable was Errett Deck, former deputy director of the Washington State Department of Agriculture (WSDA). Deck and his wife Evelyn tirelessly championed a bill through the 1988 state legislature to allow the WSDA to establish a Waste Pesticide Identification and Disposal Program. The bill granted the Department $234,000 to collect and properly dispose of unusable pesticides held by persons regulated by the state's Pesticide Application and Control Acts. An essential provision of the bill was that the Department would take legal possession of the unusable pesticide, become the "hazardous waste generator" of record and assume long-term liability for the pesticides. "Errett decided the program was needed and did the legwork to make it happen" said Lee Faulconer, current policy advisor to the Director of Agriculture, and a member of the task force that led to the creation of the program. Following passage of the legislation, Faulconer helped develop the program's operational rules and became the first coordinator of the program.
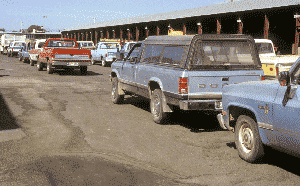
The program’s initial focus was farm properties, where it was suspected that the greatest accumulations of old pesticides were stored. The first collection event in Washington State was held in Yakima in August 1988. WSDA requested that participating individuals pre-register. Little did they know how crucial that pre-registration would be. Fifty agricultural producers signed up in advance, but 101 showed up, turning in more than 21 tons of unusable pesticides. There were long lines, with many participants waiting two to four hours. Some left in frustration. Based on sign-ups, the contractor had only four personnel on site. They worked three days after the event to process, analyze, and package three semi truckloads of waste pesticides.
First Collection Event Brings Nearly Overwhelming Response |
|
 |
 |
| Fifty customers signed up for the first event at Yakima. Unexpectedly, over 100 vehicles showed up which created two to four hour wait times. To prevent long waiting lines, the program now schedules groups of customers into time periods and only discloses the site location to those who sign up. | WSDA and Chem Pro Environmental were overwhelmed by over twenty-one tons of old pesticides at the first event in Yakima in 1988. |
After that first event (which nearly exhausted the funds that had been appropriated for that year), it became obvious that there were more than 86,000 pounds of waste pesticides to be disposed of in Washington. It also became obvious that advance sign-ups weren’t just a good idea, they needed to be mandatory. Today, sign-ups and inventories are required, and the exact locations of the collection events are not publicized; only those who have signed up receive the location information and map. Some states are able to do a “come on down!” type of public event, but this requires a budget more substantial than Washington State’s.
In the fall of 1988, Washington State voters passed Citizens Initiative 97, establishing the pioneering Model Toxics Control Act. One of the provisions of the act was the establishment of the Model Toxics Control Account to begin cleaning up hazardous waste problems around the state and to help prevent future contamination. The Waste Pesticide Program has received legislative appropriations of approximately one percent from this account since then to continue disposing of the backlog of unusable pesticides and to help prevent future accumulations. This fund has also been instrumental in addressing and capping old, poorly designed city and county landfills and cleaning up toxic waste sites, including methamphetamine labs. It also helped establish a household hazardous waste program in every county within the state.
Growth and Maturity of the Programs
Many other states benefited from Deck's and Faulconer's work in Washington, using our state’s program as a framework to design and establish their own disposal programs. As a result of the successful programs initiated by many states, EPA established the Universal Waste Rule in 1995, which eliminated many of the regulatory hurdles and increased flexibility to streamline existing programs and help start additional ones.
In Washington, the program has collected pesticides from all corners of the state and everywhere in between. In addition to disposal of excess stocks, the program has responded to pesticides made unusable due to damage from floods, fires, and the Nisqually Earthquake. In May 2000, the WSDA Waste Pesticide Program collected and properly disposed of its one millionth pound. The program has disposed of 1,382,052 pounds from 4,420 customers as of December 2002. Last year set a calendar-year record of 171,713 pounds collected. The previous high was 152,237 pounds in 1999.
The program is working to keep up with continual product cancellations. While it still collects DDT and other long-canceled pesticides, an increasing percentage of the waste pesticide mix comes from those pesticides being dropped as part of the re-registration process and cancelled as a result of the Food Quality Protection Act of 1996. The four pesticides collected in the largest amounts have been dinoseb, DDT, 2,4-D, and endrin. With the exception of some formulations of 2,4-D, these pesticides were cancelled over the last thirty years. Malathion, parathion, sulfur, captan, zineb, and pentachlorophenol round out the top ten. Many of the pesticides disposed are over twenty years old. The oldest verifiable waste pesticide collected to date by the program was lead arsenate from 1913.
The Top Four Pesticides Collected in Washington |
|
 |
 |
| More pounds of Dinoseb, the active ingredient in Vertac General Weed Killer, have been collected than any other single pesticide ingredient. | The lid crimping process scratched the paint which allowed rust to commonly destroy the lids on old five-gallon cans such as this DDT Flowable. |
 |
 |
| The label on this 1950s era container of 82.5% 2,4-D stated, "This product should not be used on rangeland until more is known about this new and promising herbicide." | In retrospect, it was very dangerous to package highly toxic and persistent Endrin 1.6 EC in glass bottles. Endrin became a Restricted-Use pesticide before its use cancellation in the mid-1980s. |
Note that WSDA does not simply send the waste pesticides they collect to landfills. Most go through hazardous waste incineration. Those that cannot be destroyed by high heat are treated in another manner. For example, lead arsenate is stabilized through encapsulation then consigned to a landfill; mercury is sent off for retort and reclamation.
Program Participation Benefits
What does this service do for the individual pesticide user? For one thing, it saves them money. LOTS of money; safe pesticide disposal is an expensive proposition. Not only does WSDA provide the service free of charge (they achieve a substantial per-pound discount due to the volume of pesticides they collect), they also bear the expense of various registrations and document maintenance required for legal disposal. Most individuals find they need to hire a contractor to deal with the paperwork involved just to set up and implement disposal on their own. Once the time and money has been expended to establish a waste pesticide collection transaction, the cost for disposal of, for example, a 55-gallon drum of a pesticide such as 2,4-D, would be around $600. This figure excludes set-up fees, travel beyond an established waste collection site, and any special handling and packaging that might be necessary. Note that a small amount of pesticide (e.g., a five-gallon can) can cost almost the same as a drum.
Another benefit, less tangible but extremely important, is that WSDA assumes the legal responsibility as the generator of the waste it collects at all collection events. The participating individual’s name is listed nowhere in the documentation. Participating in this program literally removes long-term liability from the individuals who bring their waste for disposal.
In the case of especially toxic chemicals, there is one more advantage: safety. Some of the waste pesticides disposed in the WSDA program have been downright lethal. Leaving such chemicals on the farm or business site could prove hazardous to loved ones and employees. The investment in time required to contact WSDA and inventory one’s pesticides prior to a collection event is minimal when the financial, legal, and safety aspects are considered.
Washington’s Program Today
WSDA’s Waste Pesticide Program is open to anyone who has unusable agricultural and commercial grade pesticides. Most of the pesticides are brought to collection events by individuals who have signed up and submitted inventories of the pesticides they wish to dispose. The pesticides are turned over to a hazardous waste contractor who packs them for safe transportation and destruction. WSDA oversees the collection, pays the hazardous waste disposal fees, and, as mentioned above, accepts long-term liability. In some cases, people cannot identify or for some other reason are unable to inventory their pesticides. These might be individuals who have purchased a property and have found an accumulation of old pesticides or those whose health and/or lack of pesticide knowledge hampers their ability to sort through the pesticides and deliver them to a collection event. Upon request, staff will come to a customer's site at no charge to help them sort through their pesticides and package them for proper transport. Since many of these old pesticides are highly toxic and/or persistent, people without proper knowledge or training should leave them alone and call an expert for help. In addition to program staff, local agricultural pesticide dealers, university Cooperative Extension agricultural specialists, and crop advisors are an excellent source of information and can help a person sort through their pesticide stock and determine those that can no longer be used.
Interested persons can contact the program by email at wastepesticide@agr.wa.gov, by telephone toll free at 1-877-301-4555, by mail at WSDA Waste Pesticide Program, PO Box 42589, Olympia, WA 98504-2589, or visit the Website at http://www.wa.gov/agr/PestFert/EnvResources/WastePesticide.htm.
Joe Hoffman is the Program Coordinator for WSDA’s Waste Pesticide Program.
We're from the Government, and We Really ARE Here to Help! |
|
 |
 |
| Who knows what may lurk in an old, remote outbuilding? Well over one ton of quart bottles of the old insecticide Letholol were removed from this shed. | Collection events have come a long way since the first one in Yakima. The sign-up and inventory process greatly reduces customer wait times and allows for a safe and orderly collection event. Three vehicles are being unloaded simultaneously at this 1994 event held at the WSU Prosser facility. WSU has loaned many of their sites around the state for collection events. |
Upcoming WSDA Waste Pesticide Program Collection Dates |
||
SPRING |
||
| Nearest City | Event
Date |
Sign-Up*
Before |
| Puyallup | 29-Apr |
17-Mar |
| Yakima | 6-May |
24-Mar |
| Spokane (Colbert) | 8-May |
25-Mar |
| Summer
and Fall dates are tentative, depending upon continued program funding. |
||
SUMMER |
||
| Mount Vernon | 19-Aug |
17-Jul |
| Seattle | 20-Aug |
17-Jul |
| Centralia | 21-Aug |
18-Jul |
| Vancouver | 22-Aug |
18-Jul |
FALL |
||
| Pullman | 16-Sep |
29-Jul |
| Dayton | 17-Sep |
29-Jul |
| Pasco | 18-Sep |
29-Jul |
| Wenatchee | 7-Oct |
18-Aug |
| Okanogan | 8-Oct |
18-Aug |
| Oroville | 9-Oct |
18-Aug |
*Signing up and submitting an advance inventory is REQUIRED for participation. Inventory forms will be sent to you when you sign up. In situations where the owner is unable to identify the pesticide, or needs assistance with packaging or inventorying their unusable pesticides, WSDA may be able to provide on-site assistance or to refer the inquiry to another resource for this service. Contact WSDA at wastepesticide@agr.wa.gov or 1-877-301-4555. |
||
Salmon-Stimulated Lawsuits
Swimming in Circles or Shouldered on Sound Science?
Dr. Allan S. Felsot, Environmental Toxicologist, WSU
During July 2002, the U.S. District Court of Western Washington (i.e., the Federal Court) issued a summary judgment declaring that EPA was in violation of the Endangered Species Act (ESA). The judgment resulted from a lawsuit filed by a consortium of advocacy groups (i.e., the Consortium) that declared the EPA failed to consult with the National Marine Fisheries Service (NMFS) during the pesticide registration process. Fresh from its victory, the Consortium filed a motion in November 2002 for injunctive relief from the use of 54 pesticides listed in the Federal Court’s summary judgment (Table 1). In short, the injunction is requesting restrictions on the use of the pesticides nearby water bodies that may harbor salmon.
At stake is the ability of growers to continue to use pesticides in a manner that has followed the guidelines approved by the EPA to ensure the mandates of FIFRA (Federal Insecticide, Fungicide and Rodenticide Act). A 1972 amendment to FIFRA known as FEPCA (Federal Environmental Pesticide Control Act) requires EPA to determine a reasonable certainty of no harm to the environment before registering a product. The original lawsuit and following motion for injunction imply that the reasonable certainty standard has been violated when endangered salmon populations are considered.
Considering the intense scrutiny
that EPA puts into assessing the human and environmental hazards from
pesticides, lawsuits declaring the process inadequate and therefore seeking
remedies bear closer examination for their scientific merits. With regard
to salmon, two questions come to mind. Are salmon populations really in
danger from pesticide residues at concentrations found in the environment,
and will the requested injunctive relief actually protect salmon? After
all, if hazards to salmon from pesticide residues are negligible and the
restrictions requested in the injunction are no more protective than current
practices, why impose them at all?
TABLE 1 |
|||
The
54 pesticides cited in the summary judgment by the Federal Court
that requires EPA to consult with NMFS about their effects on protected
salmon populations. Darker boxes (items in white type) indicate
that EPA has already issued a risk assessment specifically for listed
salmon populations and is in the process of consultation with the
National Marine Fisheries Service (EPA 2002a). |
|||
| Pesticide | Use
(1) |
Pesticide | Use |
| 2,4-D | H |
Acephate | I |
| Alachlor | H |
Azinphos-methyl | I |
| Atrazine | H |
Carbaryl | I |
| Bensulide | H |
Carbofuran | I |
| Bentazon | H |
Chlorpyrifos | I |
| Bromoxynil | H |
Coumaphos | I |
| Dicamba | H |
Diazinon | I |
| Dichlobenil | H |
Diflubenzuron | I |
| Diuron | H |
Dimethoate | I |
| Linuron | H |
Disulfoton | I |
| Metolachlor | H |
Ethoprop | I |
| Metribuzin | H |
Fenamiphos | I |
| Molinate | H |
Lindane | I |
| Norflurazon | H |
Malathion | I |
| Oryzalin | H |
Methamidophos | I |
| Oxyfluorfen | H |
Methidathion | I |
| Paraquat dichloride | H |
Methomyl | I |
| Pebulate | H |
Methyl parathion | I |
| Pendimethalin | H |
Naled | I |
| Prometryn | H |
Phorate | I |
| Simazine | H |
Phosmet | I |
| Tebuthiuron | H |
Thiodicarb | I |
| Terbacil | H |
||
| Thiobencarb | H |
Fenbutatin-oxide | A |
| Triallate (2) | H |
Propargite | A |
| Triclopyr | H |
||
| Trifluralin | H |
||
| 1,3-Dichloropropene | F |
||
| Captan | F |
||
| Chlorothalonil | F |
||
| Iprodione | F |
||
| (1) H = Herbicide; F = Fungicide; I = Insecticide; A = Acaricide | |||
| (2) Triallate was not included in the summary judgment over EPA’s failure to consult with NMFS but was assessed individually by EPA for effects on listed salmon. | |||
Legal History
Currently, salmon populations known as Evolutionarily Significant Units (ESUs) have been declared endangered or threatened under the Endangered Species Act (ESA). The ESA mandates that ESUs receive Federal protection. As almost all of the 26 declared salmon ESUs are present exclusively in the Pacific Northwest, the impact here is great. In addition to disallowing all kinds of activities that may harm the ESUs, the ESA directs Federal agencies to consult with the NMFS to insure that agency actions do not jeopardize the ESUs. The consultation provision states that each agency shall use the best scientific and commercial data to make decisions about protection.
The ESA also allows citizen-initiated lawsuits against any person, group, business, or governmental agency that is perceived to be violating the ESA’s provisions to protect threatened and endangered species. Taking advantage of this democratic provision, the Consortium sued EPA under two mandates of the ESA known as Section 7(a)(1) and 7(a)(2). The Consortium reasoned that the EPA in its re-registration of pesticides used in the PNW violated the consultation provisions of the ESA (the Section 7(a)(2) complaint). Furthermore, the Consortium claimed that EPA failed to use its authority in consultation with NMFS to promote the conservation of the salmon ESUs (the Section 7(a)(1) complaint).
In July 2002, the Federal Court issued a summary judgment that agreed with the premise of the Consortium’s lawsuit regarding EPA’s failure to consult with NMFS in its agency actions to re-register pesticide active ingredients (the Section 7(a)(2) complaint). However, the Court ruled that only 55 pesticides (54 individual active ingredients had actually been identified, Table 1) were covered by the summary judgment because the Consortium failed to provide the identity and evidence of harm for any of the 898 other registered active ingredients.
Regarding the Consortium’s Section 7(a)(1) complaint, the Court found that EPA had taken actions to conserve salmon ESUs and had indeed shown effort to consult with NMFS on pesticide management practices.
The purpose of the summary judgment was not to rule on the merits of whether the 54 pesticides indeed posed a threat to salmon ESUs. However, in ruling that the 54 pesticides were subject to the Section 7(a)(2) consultation provision of the ESA, the Court noted the plaintiffs (i.e., the Consortium) submitted “scientific or competent declaratory evidence demonstrating a causal link between EPA’s ongoing registration actions and direct or indirect adverse effects on salmonid populations.” According to a footnote in the summary judgment, the Court considered EPA’s own reports as sufficient documentation of potentially significant risks to salmon posed by certain registered pesticides.
The summary judgment is unclear as to whether the Federal Court had considered scientific arguments counter to the perspective of pesticide harm on listed salmon populations, but the merit of the lawsuit rested solely on whether EPA violated the consultation provision of ESA. However, the summary judgment recognized the Consortium’s argument that “their burden with respect to causation [i.e., of harm to salmon] is minimal.”
EPA is Reactive and Proactive
In response to the Court’s summary judgment, EPA has been issuing findings on potential risk of 54 pesticides to specific salmon populations listed for ESA protection. EPA has established a Web site to issue their determination of potential harm or no harm and has opened the opinions to public comment (EPA 2002a). EPA is just beginning consultations with the NMFS on potential risk of the earmarked pesticides to salmon and/or their habitat. EPA has also implemented an Endangered Species Protection Program (ESPP) and is currently seeking public comments about its plan (EPA 2002b).
The stated goal of the ESPP is to “carry out responsibilities under FIFRA in compliance with the ESA while at the same time not placing unnecessary burden on agriculture and other pesticide users.” In addition to describing how consultations with agencies administering the ESA (i.e., NMFS and the Fish and Wildlife Service [FWS]) would take place, the ESPP describes how EPA will meet its responsibilities under Section 7(a)(1) of the ESA by completing and upgrading County Bulletins, amending pesticide labels to reference County Bulletins, and enhancing monitoring programs. County Bulletins describe the locations of protected ESUs and restrictions on registered pesticides that may be used in those areas. The bulletins can be accessed at http://www.epa.gov/espp.
The Consortium Strikes Again
Not content with EPA’s response or its ESPP, the Consortium filed during November 2002 a motion for injunctive relief. In brief, the Consortium is requesting that the Federal Court impose use restrictions on the 54 pesticides covered in the summary judgment regarding violations of EPA’s consultation responsibilities. The rationale for the request is that the ESA specifically requires that its administering agencies (NMFS and FWS) issue a comprehensive biological opinion on protected ESUs prior to initiation of any agency (e.g., EPA) action. Each pesticide registration would be considered a single agency action.
Because the 54 subject pesticides are still registered and therefore legal to use in the watersheds containing the affected salmon habitats, the EPA has essentially taken “action” prior to NMFS issuing a biological opinion. Thus, to remedy this violation and protect salmon prior to completion of consultations, the Consortium wants the Federal Court to issue an injunction prohibiting EPA from continuing to authorize use of the 54 pesticides by ground spraying within 20 yards (~60 ft) or by aerial spraying within 100 yards (~300 ft) of water bodies accessible to listed salmon. Furthermore, the Consortium is requesting that the Federal Court prohibit use in urban watersheds of a subset of 13 pesticides unless they are sold by a licensed pesticide dealer and applied by a certified pesticide applicator. In short, the Consortium is requesting the establishment of no-spray buffer zones around streams when pesticides are used in agriculture, buffer zones along rights-of-way, and prohibition of homeowner use of certain pesticides.
Where’s the Science?
Because the Federal Court had not ruled previously on whether the 54 pesticides harm salmon, this discussion need not lapse into a food fight over the merits of the Court’s summary judgment mandating EPA to consult with NMFS. However, in making its case for an immediate junction to proscribe use of the named 54 pesticides, the Consortium claims to have presented scientific evidence of immediate harm to salmon. The evidence comes in two forms: (1) the USGS has detected pesticides in water of the Pacific Northwest that are associated with adverse effects on salmon or their habitat; (2) the EPA has characterized ecological risk of many of the 54 pesticides as exceeding their levels of concern (LOCs) for fish and their habitat.
Scrutiny should be brought to bear on claims that residues detected by USGS are associated with adverse effects on salmon or their habitat. Two measures of toxicity are used to determine potential adverse effects (or hazard): acute and chronic. Acute tests determine the concentration lethal to 50% of the test population after exposures for 48-96 hours. Results are expressed as an LC50. With regard to judging potential effects on populations, the LC50 has a lot of merit because wiping out 50% of breeding individuals has a reasonable probability of hurting the reproductive potential of a population. Chronic toxicity is measured after exposing a test population to a pesticide throughout its breeding life cycle. To describe this effect, the NOAEC (No Observable Adverse Effect Concentration) is used; the chronic test represents a direct measurement of possible harm to reproductive potential. In these tests, measurements on fish growth in addition to effects on reproduction can be used as an adverse effects endpoint.
Concentrations of pesticides detected in the USGS studies of Oregon and Washington watersheds are lower than known lethal susceptibilities of salmon (Bortleson et al. 2000; Rinella and Janet 1998; Voss et al. 1999; Williamson et al. 1998). For example, azinphos-methyl (AZM) has been tested on salmon and has been found to be the most toxic of orchard pesticides used in the Pacific Northwest (EPA 1999). The LC50 for coho salmon is 3.2 ppb, but the highest detection was only ~0.5 ppb and occurred in an agricultural drain in the Columbia Basin where AZM use would be intense (Williams et al. 1998). More importantly, the USGS found AZM in only 12% of the collected samples in the Basin, and almost all were below 0.1 ppb. Nationally, 95% of samples contained less the 0.002 ppb AZM (Larson et al. 1999). The USGS’s national database has a lot of relevance for AZM residues in the PNW because the insecticide is mostly used in pome fruit production.
Possible habitat effects can be considered as changes in abundance of food resources. Invertebrates like the microcrustacean Daphnia serve as surrogate species. Daphnia reproduction was not affected by a concentration of 0.25 ppb (which is the NOAEC). Logically, concluding that a compound like AZM is harming salmon directly or indirectly doesn’t make sense when 90% of water samples contain no detectable amounts, there is a 30-fold safety factor between the acute LC50 and almost all detected concentrations (i.e., the ratio of 3.2 ppb to 0.1 ppb), and reproduction of the most sensitive invertebrate is likely not affected by the environmental concentrations.
The Consortium has made additional
arguments that pesticides found by the USGS have sublethal effects that
harm salmon. This conclusion seems to come from literature studies of
diazinon’s potential to modify olfactory responses of salmon to
anti-predatory alarm pheromone and sex pheromone (Mooring and Ware 1996;
Scholtz et al. 2000). I have previously discussed my analyses of these
data (Felsot 2001d) and concluded the actual concentrations found in the
PNW are too low for the purported effects on salmon olfaction to be ecologically
relevant. Similarly, I have previously argued that sublethal effects of
pesticides on fish have not been shown to occur at concentrations actually
found in the environment (Felsot 1999a).
Ecological relevance of pesticide residues on salmon populations in itself
bears some examination. I have surmised that part of the argument over
harm represents confusion between effects on individuals versus effects
on populations. From an ecological perspective, harming a population would
entail serious decline in reproductive potential that prevented the population
from maintaining a sustainable size. Removing a few individuals would
not harm the population as long as reproductive potential was maintained.
After all, if harming individual salmon were problematic, NMFS would have
to eliminate fishing.
In contrast to the potential harm hypothesized for pesticides, fishing definitively has killed salmon. Thus, the claims that currently registered pesticides harm salmon populations by either direct mortality or sublethal effects or by effects on their food sources is specious because such effects, if they are occurring at all, have never been shown to affect reproductive potential of the protected salmon ESUs. As I’ve just argued for AZM, the extent of occurrence and concentrations of pesticides in the PNW are just too low to have any direct or indirect effect on salmon populations, even if some individuals were sublethally affected.
EPA Shoots Itself in the Foot
The second argument used by the Consortium to bolster its case for an injunction is that EPA itself has concluded that registered uses of the 54 pesticides “would likely result in environmental contaminations that exceed EPA’s levels of concern for fish and habitat.” The Consortium has interpreted EPA’s risk characterization as being applicable to salmon. EPA reports its ecological risk characterizations in a pesticide’s registration eligibility decision document (RED). However, EPA does not use these documents to discuss specific endangered species, but only to conclude whether the level of concern (LOC) established for endangered species in general is exceeded.
But what does it really mean to exceed an LOC? The Consortium’s injunction argument seems to ignore how the LOC is determined. For example, they state, “Based on toxicity studies, EPA establishes regulatory levels of concern for fish, aquatic invertebrates, and aquatic plants.” In fact, an LOC is a benchmark value not to be exceeded when one calculates the ratio of the expected (or estimated) environmental exposure (EEC) relative to a toxicological endpoint (i.e., the LC50 or the NOAEC) for the most sensitive organism. This ratio is called the risk quotient (RQ). The LOCs for RQs that are not to be exceeded for different risk presumption scenarios are shown in Table 2. Note that EPA protects endangered species by setting the LOC for acute toxicity to a conservative level of 0.05. However, for chronic toxicity, which measures adverse effects on growth and/or reproduction, the NOAEC should not be exceeded. Whatever the basis for determining the tolerable LOC, bear in mind that its value is not based on any empirical scientific evidence of harm but rather a risk management decision to prevent harm.
TABLE 2 |
|||
| EPA
Risk Characterization Guidelines
(see Felsot et al. 1999a) |
|||
| Risk
Presumption Category |
Risk
Quotient Calculation (1) |
Level
of Concern |
Effective
Safety Factor |
| Acute High Risk | EEC/LC50 |
0.5 |
2 |
| Acute Restricted Use | EEC/LC50 |
0.1 |
10 |
| Acute Endangered Species | EEC/LC50 |
0.05 |
20 |
| Chronic Risk | EEC/NOAEC |
1 |
1 |
| (1) EEC = estimated environmental concentration; LC50 = concentration of pesticide lethal to 50% of test subjects in 96 hours; NOAEC = no observable adverse effect concentration | |||
An important part of the RQ is exposure information; the way that information is obtained influences perception of risk. For almost all pesticides registered or re-registered to date, EPA uses computer models to estimate pesticide residues in aquatic habitats. The computer models, named PRZM (Pesticide Root Zone Model) and EXAMS (Exposure Analysis Modeling System) simulate particular crop uses, application rates, weather conditions, degradation rates, and drift potential. The target body of water is a fixed pond two acres in area by six feet deep. For many pesticides, degradation in water is considered much slower than in soil, so once the pesticide gets to water, it would be modeled to stay around quite a while during the growing season. As a result, initial and chronic (over 60-90 days) residues are much higher in EPA’s modeling exercises than reported from the agricultural streams monitored by the USGS. More important is the fact that salmon live in flowing streams, not static ponds. Concentrations of pesticides would quickly become diluted under flowing conditions. Thus, claiming that EPA’s own risk characterization indicates harm to salmon indicates an uncritical acceptance that EPA’s method of deciding exposure is even relevant to salmon in the first place.
The One-Size-Fits-All Approach Is Illogical
Although I have been skeptical that pesticides used in agricultural watersheds throughout the PNW are directly or indirectly harmful to salmon populations, I am a supporter of the use of buffer zones to protect sensitive organisms (whether they be people, plants, or salmon) (see Felsot 1999b). However, I am not supportive of the one-size-fits-all approach requested in the Consortium’s injunction. Rather, I have argued that buffer zones should be based on toxicological relevance and the specific environment of operation rather than set as a singular distance applying to all situations.
The first reason I am against singular buffer zone widths is because the 54 pesticides in question represent a mix of herbicides, fungicides, insecticides, and acaricides (Table 1). These chemicals have a myriad of toxicological modes of action as well as varying toxicities against sensitive fish and invertebrate species (Figure 1). Generally, the herbicides and fungicides are much less toxic to aquatic organisms than the insecticides or acaricides. Therefore 1 ppb of herbicide in water will have a much different hazard potential for invertebrates than 1 ppb of an organophosphorus (OP) insecticide.
FIGURE 1 |
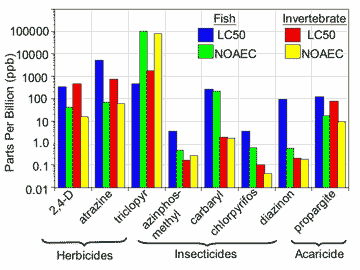 |
| Toxicological endpoints for selected types of pesticides cited in the summary judgment of the U.S. District Court of Western Washington. Examples were chosen to represent different kinds of pesticides and different levels of hazard; residues of the chosen compounds have been detected in the waters of the Pacific Northwest. Pesticides with longer bars are comparatively less hazardous. Note that the insecticides tend to have much shorter bars than the herbicides and the acaricide, especially for invertebrates. Toxicological values were derived from information in each pesticide’s registration eligibility decision document. |
The second reason I am against singular buffer zone widths is because each agricultural field is unique. For example, fields in the Columbia Basin and Yakima Valley of eastern Washington do not experience runoff because most are now irrigated by moving sprinklers or drip systems. Thus, a buffer zone designed to slow down runoff seems useless. On the other hand, a no-spray buffer set to reduce direct drift in water should take into account realistic conditions in combination with concentrations likely to cause no harm. If one compound is less hazardous to aquatic organisms than another compound, equally sized no-spray buffers do not increase safety.
Putting the Proposed No-Spray Buffer Zones to the Test
The Consortium argues in its injunction request that its proposed no-spray setbacks from water bodies will prevent harm to salmon in the interim between EPA consultation with NMFS and the issuance of a biological opinion. The Consortium seems to be making the assumption that spray drift may be the most significant pathway to aquatic habitat contamination. To determine whether these proposed buffer zones were indeed protective or perhaps overkill, I used the AgDrift model and realistic pesticide use scenarios to estimate residues in water at various distances from crop rows at the field edge.
The AgDrift model is one tool being used by the EPA to estimate the magnitude of drift during spraying (Teske et al. 2002). AgDrift’s aerial application module is partly theoretical because it relies on the physics of particles to estimate their downwind movement potential. The module for ground spraying is largely empirical because it relies on the results of numerous experiments to estimate drift potential. Another module of AgDrift can estimate residues in a flowing stream over time, so that the dilution of depositing pesticide residues can be considered (Teske et al. 2001).
I chose two compounds from the list of 54, AZM and propargite. AZM is usually applied from the ground using an airblast sprayer that directs the sprays up into the tree canopy. Propargite is used on alfalfa seed crops, hops, mint, and potatoes. It can be applied through an irrigation system (on potatoes only) or by ground or aerial application. The choices of AZM and propargite, therefore, allowed realistic simulations of an orchard application by ground and a row crop application by air. Furthermore, AZM was chosen because it represented an OP insecticide tested directly on coho salmon and it is one of the most acutely toxic insecticides to fish. Propargite is not particularly toxic to fish, but the EPA did hypothesize that some ESUs in the Pacific Northwest may be affected by residues of the acaricide.
For each compound, I chose a more conservative aquatic target than the EPA uses. My target was a 9.84-ft wide by 1.64-ft deep stream. In the first modeling scenario I treated the stream as a pond (i.e., the water was static) and modeled an airblast and airplane sprayer moving along a crop row that was 328 feet long. Thus, the stream would be receiving spray drift along this entire length. The area of the receiving “pond” with my stream’s dimensions therefore was 0.07 acres compared to EPA’s two-acre pond. Choosing a shallow, narrow stream is conservative because its significantly lower volume in comparison to a 6-ft deep pond will result in much higher pesticide concentration following a constant amount of drift.
I purposefully chose the particular dimensions of the stream to coincide with the dimensions of the default stream used by AgDrift to estimate residues in a flowing system. For flowing stream assessment, I used the model’s default flow rate of 2.24 mph. I asked the model to tell me what concentrations to expect in the stream over a period of 15 minutes at downstream locations of 0, 10, 100, and 1000 feet. The no-spray buffer zone was 60 ft for the airblast sprayer and 300 feet for the aerial application.
And the Winner Is…
After modeling the percentage deposition of spray drift onto the water, I assumed instantaneous mixing throughout the water column and therefore used the volume to change the residues into ppb. The results from modeling spray drift into a shallow and narrow non-flowing stream showed that the size of the buffers needed to protect salmon against the acute effects of pesticides are substantially smaller than the no-spray setbacks proposed in the Consortium’s request for an injunction. For example, Figures 2 and 3 show the residues in water expected from deposited spray drift of AZM and propargite, respectively, using different setback distances. Overlain on these curves are the toxicologically relevant endpoints for fish and invertebrates.
FIGURE 2 |
 |
| Azinphos-methyl residues in water of a non-flowing “stream” simulated using the model AgDrift. The pesticide was sprayed at a typical rate of 1 lb AI/acre from an airblast sprayer moving parallel to the water body. Spraying was simulated on twenty rows of trees and the outside or last tree row was assumed to be at increasing distances from the edge of the stream. The red and brown lines represent the pesticide concentration corresponding to the ecotoxicological endpoints. The green line represents the no-spray buffer zone distance of 60 feet. The dashed vertical arrow represents the distance corresponding to a risk quotient of 0.1 (i.e., the residues in water are ten fold less than the coho salmon LC50). The solid black arrow represents the no spray buffer zone distance (~115 ft) where the depositing residues in water would be equivalent to an RQ of 0.05 (EPA’s level of concern for endangered species). |
FIGURE 3 |
 |
| Propargite residues in water of a non-flowing “stream” simulated using the model AgDrift. The pesticide was sprayed from an Air Tractor AT401 fixed wing aircraft moving parallel to the water body. Spraying was simulated at a maximum label rate of 1.88 lbs AI/acre Comite on twenty rows of potatoes and the outside or last spray swath was assumed to be at increasing distances from the edge of the stream. Other model input parameters were 70 deg F, 75% relative humidity, 4 mph cross wind. The red and brown lines represent the pesticide concentration corresponding to the ecotoxicological endpoints. The green line represents the no-spray buffer zone distance of 300 feet. The dashed vertical arrow represents the distance corresponding to a risk quotient of 0.1 (i.e., the residues in water are ten fold less than the rainbow trout LC50). The solid black arrow represents the buffer zone distance where the depositing residues in water would be equivalent to an RQ of 0.05 (EPA’s level of concern for endangered species). |
A setback distance of 40-45 feet was simulated to cause AZM water residues that were 10 times less than the LC50 for coho salmon and equal to the fish NOAEC (Figure 2). For propargite, the setback distances giving tenfold protection from acute toxicity to rainbow trout (which is in the same genus as coho salmon) and equivalence to the NOAEC for growth effects was 59-73 feet (Figure 3). Thus, the buffers based on drift modeling and the protection of salmon from acute toxicity are less than the 60 ft buffers requested for a ground spray of AZM and the 300 ft buffer requested for an aerial spray of propargite.
A no-spray setback distance of 91-105 ft should be sufficient to protect invertebrates from acute and chronic toxicity of propargite. The no-spray setback to protect aquatic invertebrates from chronic toxicity of AZM would be 60 ft, but over 650 ft would be required to provide a 10-fold safety factor for acute toxicity (i.e., the concentration of AZM would have to be 0.016 ppb).
The previous analysis simulating a non-flowing stream segment would be most appropriate for protecting endangered frogs living in ponds of fixed volume and no inlet or outlet. However, if we’re really interested in protecting salmon, exposure should be simulated in the appropriate habitat, a flowing stream. When AgDrift is used to generate the rise and fall of pesticide residues along a 1000 ft length of stream, the picture of potential exposure changes entirely from that in a fixed volume of water. Figures 4 and 5 show that residues in flowing water that drifted from a ground sprayer using a 60-ft no-spray buffer or an airplane using a 300-ft buffer should peak within 50-60 seconds after application and then fall quickly to very low levels within 10-15 minutes. For example, at a downstream distance of 10 ft, AZM residues peaked at a concentration 0.07 ppb and then dropped to ~0.01 ppb within 13 minutes. Using a 300-ft no-spray buffer, aerial application of propargite resulted in maximum residues of 1 ppb within 50-60 seconds that then dropped to zero in about 5 minutes. The later occurring peak in residues observed after 5 minutes represents a ‘wave’ of residue traveling from upstream locations. Modeling flowing stream residues with a no-spray buffer of 50 ft gave higher initial deposits (for example, 20 ppb for propargite), but residues declined to concentrations below 1 ppb within minutes.
FIGURE 4 |
 |
| AgDrift model simulation of azinphos-methyl residues in a stream flowing at 2.24 mph following an airblast ground application to 20 rows of an orchard and a no-spray buffer zone of 60 ft. The spray line was 328 ft long, and the stream was 9.84 ft wide by 1.64 ft deep. |
FIGURE 5 |
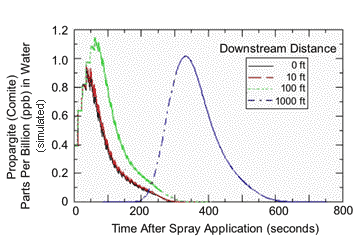 |
| AgDrift model simulation of propargite residues in a stream flowing at 2.24 mph following an aerial application to 20 rows of a potato field and a no-spray buffer zone of 300 ft. The spray line was 328 ft long, and the stream was 9.84 ft wide by 1.64 ft deep. Other input parameters were the same as in Figure 3. |
Protecting sensitive invertebrates in ponds against OP insecticides would require very large no-spray buffers, which would be difficult to implement commercially. Protecting them in flowing streams requires minimally sized buffers because the water volume is changing and constantly diluting the insecticide. If I had modeled herbicide applications, no-spray zones for fixed ponds could be even smaller than for insecticides and still achieve a reasonable certainty of no harm.
But Is It Good Enough?
EPA is in the process of making specific determinations as to whether any of the 54 subject pesticides may specifically harm salmon populations. Thus far, EPA has issued an analysis of 11 pesticides (Table 1). A “may affect” determination on one or more salmon ESUs has been made for the pesticides bensulide, diazinon, fenbutatin, metolachlor, propargite, and prometryn. Whether a pesticide will pass or fail depends on the set of exposure data that EPA uses in the risk assessment and the risk management objectives. For example, in the currently posted “may affect” determinations, runoff modeling data remains the primary means of exposure analysis. However, as I have shown, if spray drift management is the key to protecting salmon, as it is likely to be in eastern Washington or other irrigated agricultural regions, the stream assessment module of AgDrift is a more realistic measure of exposure because salmon live in flowing waters.
Nevertheless, as long as EPA maintains the necessity for an RQ for acute toxicity no greater than 0.05 (which is equivalent to a 20-fold safety factor based on the LC50) to protect endangered species, the most hazardous organophosphorus insecticides like AZM will need buffer zones even larger than sought by the Consortium (~115 ft would be required, Figure 2). In essence, the question of harm to salmon may hinge just as much on appropriate safety factors (a social issue) as much as on arguments over appropriate residue data to use in risk characterization (a scientific question).
Growers Lead the Way to Protect Salmon
While all the lawyers are arguing in court about how to best help salmon, growers and even chemical manufacturers are making the changes that will fix the ESA-driven concerns over harm from pesticides. With regard to the most hazardous compounds, the OP insecticides, registrants DowAgroSciences and Syngenta have voluntarily removed from market the popular home and garden pesticides chlorpyrifos and diazinon. Ironically, agriculture contributed very little to the load of these pesticides in comparison to urban uses (Larson et al. 1999). Because these are the most frequently detected insecticides in water, the hazards to salmon, if any, should eventually approach zero.
With regard to the remaining few uses of OP insecticides in agriculture, the FQPA as well as grower desires to adopt IPM systems and save on pesticide costs has stimulated big changes in use patterns. Thus, use of AZM, perhaps the most widely used fruit insecticide, has dropped by 40% over the last decade as apple growers have increasingly adopted the use of pheromones for mating disruption and control of the codling moth (Jones 2002). As OP insecticide use becomes less of an option and manufacturers increasingly commercialize reduced risk pesticides, growers will adopt the newer chemical technologies. The only drawback is that the cost of the newer, safer products is much greater than cost of the older products. But with improved IPM practices, growers can learn how to efficiently and more cheaply use the new technologies.
Manufacturers and growers are making changes in crop protection practices that are much friendlier to the environment. Investments are needed now to help growers develop more sophisticated IPM practices and learn how to best manage the newer reduced risk products. It is a shame precious resources are being wasted defending against lawsuits that will do nothing to improve salmon populations. Rather than spend money chasing red herrings, perhaps it is wiser to invest in commercially viable IPM systems that will inevitably help salmon populations.
Allan Felsot is an Environmental Toxicologist with WSU's Food and Environmental Quality Laboratory. He can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
REFERENCES
Bortleson, G. C. and J. C. Ebbert. 2000. Occurrence of pesticides in streams and ground water in the Puget Sound Basin, Washington, and British Columbia, 1996-98. US Geological Survey Water-Resources Investigations Report 00-4118.
EPA. 1999. Azinphos-methyl, case number 0234. Environmental Fate and Effects Division Chapter for revised registration eligibility decision document.
EPA. 2002a. Endangered Species Effects Determinations and Consultations; An Interim Process for Public Input. http://www.epa.gov/oppfead1/endanger/effects/
EPA. 2002b. Endangered Species Protection Program Field Implementation. Federal Register : December 2, 2002, Vol. 67, No. 231, pp. 71549-71561. http://www.epa.gov/fedrgstr/EPA-PEST/2002/December/Day-02/p30463.htm
Felsot, A. S. 1999a. Pesticides and salmon decline: Missing link or red herring? Agrichemical & Environmental News (May) 157:8-11, 16. http://aenews.wsu.edu/May99AENews/May99AENews.htm#anchor27243
Felsot, A. S. 1999b. Boffo buffer zones. How big is big enough? Agrichemical & Environmental News (November) 163:14-18. http://aenews.wsu.edu/Nov99AENews/Nov99AENews.htm#anchor252603
Felsot, A. S. 2001c. Pesticides and salmon, part 2. Animals in drag? Agrichemical & Environmental News (April) 180:14-20. http://aenews.wsu.edu/Apr01AENews/Apr01AENews.htm#anchor5301120
Felsot, A. S. 2001d. Pesticides and salmon, part 1. Something smells fishy. Agrichemical & Environmental News (March) 179:1-8. http://aenews.wsu.edu/Mar01AENews/Mar01AENews.htm#anchor5338542
Jones, W. 2002. Pest management practices survey results. Washington State University Tree Fruit Research and Extension Center. http://opus.tfrec.wsu.edu/~wjones/Survey2000/
Moore, A. and C. P. Waring. 1996. Sublethal effects of the pesticide diazinon on olfactory function in mature male Atlantic salmon parr. J. Fish Biology 48:758-775.
Larson, S. J., R. J. Gilliom, and P. D. Capel. 1999. Pesticides in streams of the United States--initial results from the National Water Quality Assessment Program. U.S. Geological Survey Water-Resources Investigation Report 98-4222, Sacramento, CA :99 pp. Available as PDF from http://water.wr.usgs.gov/pnsp/
Rinella, F. A. and M. L. Janet. 1998. Seasonal and spatial variability of nutrients and pesticides in streams of the Willamette Basin, Oregon, 1993-95. Resources Investigations Report 97-4082-C, U.S. Geological Survey Water, Portland, OR http://oregon.usgs.gov/projs_dir/pn366/nawqa.html
Scholz, N. L., N. K. Truelove, B. L. French, B. A. Berejikian, Quinn T. P., E. Casillas, and T. K. Collier. 2000. Diazinon disrupts antipredator and homing behaviors in chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish Aquat. Sci. 57:1911-1918.
Teske, M. E., S. L. Bird, D. M. Esterly, S. L. Ray, and S. G. Perry. 2001. A User's Guide for AgDRIFT 2.03: A Tiered Approach for the Assessment of Spray Drift of Pesticides. C. D. I. Report No. 01-01, Steward Agricultural Research Services, Inc., Macon, Missouri.
Teske, M. E., S. L. Bird, D. M. Esterly, T. B. Curbishley, S. L. Ray, and S. G. Perry. 2002. AgDRIFT: a model for estimating near-field spray drift from aerial applications. Environ. Toxicol. Chem. 21(3):659-671.
Voss, F. D., S. S. Embrey, J. C. Ebbert, D. A. Davis, A. M. Frahm, and G. H. Perry. 1999. Pesticides Detected in Urban Streams During Rainstorms and Relations to Retail Sales of Pesticides in King County, Washington. USGS Fact Sheet 097-99. http://wa.water.usgs.gov/ps.nawqa.html
Williamson, A. K., M. D. Munn, S. J. Ryker, R. J. Wagner, J. C. Ebbert, and A. M. Vanderpool. 1998. Water Quality in the Central Columbia Plateau, Washington and Idaho, 1992-95. U.S. Geological Survey Circular 1144. http://water.usgs.gov/pubs/circ1144
Washington Toxics Coalition et al. v. Environmental Protection Agency. 2002a. United States District Court, Western District of Washington at Seattle, Case No. C01-132C. http://www.pesticide.org/SalmonLawsuitOrder.pdf
Washington Toxics Coalition et al. v. Environmental Protection Agency. 2002b. United States District Court, Western District of Washington. Civ. No. C01-0132C, Motion for Further Injunctive Relief. http://www.pesticide.org/SalmonInjunction.pdf
Go to this issue's Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Announcements & Upcoming Conferences
Items in this section often appear in the words of the sponsoring organization or original news release. AENews editorial staff is not responsible for the accuracy of the content.
"National Organic Agriculture Standards" Satellite Broadcast
Washington State University Cooperation Extension and the Washington State Department of Agriculture are offering a fee-based satellite broadcast focused on the National Organic Agriculture Standards and the implications they pose for Extension Agents, state and national certification staff, consultants, and others involved in the production of organic commodities. The broadcast will present the basic requirements of the USDA's National Organic Standards and describe how the new regulations affect organic producers. The program will air March 21, 2003, from 10 am to noon, Pacific time. Viewers will have an opportunity to ask questions during the broadcast. For program details and registration information, go to:
http://ext.wsu.edu/noas/

February 26, 2003
This year's Pacific Northwest Pesticide Issues Conference focuses on the issue of Agricultural Safety and Health. The conference will be held at the Yakima Doubletree Hotel on February 26, 2003, and offers recertification credit for Washington, Oregon, Idaho, and Montana.Conference goals are to meet the education and information needs of people involved in farming, forestry, greenhouse, and nursery health and safety, with a strong emphasis on pesticide issues, and to facilitate networking and information exchange among participants. Attendees will likely represent grower associations, individual growers, forestry concerns, agrichemical dealers, crop consultants, chemical companies, state and federal regulatory agencies, and university research and extension programs.
The conference is co-sponsored by WSU Cooperative Extension, U of W Pacific Northwest Agricultural Safety and Health Center, U of W School of Public Health and Community Medicine, and the Northwest Center for Occupational Health and Safety. Conference organizer Carol Ramsay can be reached at (509) 335-9222 or ramsay@wsu.edu. More information, including the latest on the conference agenda, can be found at
Fourth National Integrated Pest Management Symposium/Workshop
Indianapolis, Indiana • April 8-10, 2003
The Fourth National IPM Symposium/Workshop is an exciting opportunity to learn about the latest developments in agricultural and urban IPM and to share your IPM experiences with others. The symposium includes over 60 breakout sessions (workshop, debate and presentation formats) encompassing almost all aspects of IPM, as well as plenary speakers talking about their experiences in building alliances. In addition, several IPM-related organizations are convening their meetings before or after the Symposium making this a full week of IPM in Indianapolis.
Examples of sessions at the Symposium include:
-
Building Alliances Between IPM Practitioners and Consumers
-
Connecting with the Media/Press
-
Biorational Insecticides - Selectivity and Importance in IPM Programs
-
Federal Agencies and IPM: Improving Communication and Coordination
-
School IPM
-
New Tools for Agricultural Professionals - WeedSOFT: A New Approach in Integrated Weed Management
-
IPM for Teachers & School Students: K-12 Curricula
-
Barriers to the Adoption of Biocontrol Agents and Biological Pesticides
-
Integrated Crop Management of Greenhouse and Floricultural Crops
-
IPM and Urban Wildlife Pest Situations
-
Application and Prioritization of IPM Projects in Natural Areas
-
Images of Sustainable Agriculture: Landscapes, Pest Management and Biotechnology
-
The Future of Global IPM
-
Landscapes and Weeds
-
IPM in Organic Systems
For more information, visit http://www.conted.uiuc.edu/ipm
USDA to Hold Farm Bill Briefings
The U.S. Department of Agriculture has announced a series of public meetings entitled, "Briefing on the 2002 Farm Bill & USDA Programs and Services." Sessions are slated for February in Puerto Rico, Nebraska, California, and Washington, with more being planned for the future. The Washington session will be held
Monday, February 24, 2002
Trade, Recreation, Agricultural Centers (TRAC)
Pasco, WashingtonThe briefings are designed to highlight changes and new provisions in the 2002 Farm Bill and to acquaint USDA customers with a wide array of agency offerings. They will enable the Department to interact with a broad spectrum of customers, including small farmers, growers, landowners, municipalities, community-based organizations, and under-served customers, particularly minorities and women. We want to ensure that all of our customers benefit fully from USDA programs and services.
Participating agencies include: Agricultural Marketing Service; Agricultural Research Service; Animal and Plant Health Inspection Service; Cooperative State Research, Education, and Extension Service; Farm Service Agency; Food Safety and Inspection Service; Food and Nutrition Service; Foreign Agricultural Service; Forest Service; Grain Inspection, Packers & Stockyards Administration; USDA Office of Outreach; Natural Resources Conservation Service; Risk Management Agency; and Rural Development.
Briefings are free and open to the public. For additional information, you may contact the USDA Office of Outreach at 1-800-880-4183 or go to the Web site at
http://www.usda.gov/da/briefings/briefing.htm
Sustaining Agriculture Newsletter Launched
Announcing the Sustaining the Pacific Northwest - Food, Farm, & Natural Resource Systems quarterly electronic newsletter, jointly sponsored by the WSU Food & Farm Connections Team, WSU Center for Sustaining Agriculture and Natural Resources, and the WSU Small Farms program. Sustaining the Pacific Northwest provides a discussion forum for people working towards community-based sustainable food, farm, and natural resource systems using interdisciplinary oriented research and practitioner knowledge.
The inaugural issue can be viewed or downloaded from http://csanr.wsu.edu/whatsnew/PNW-v1-n1.pdf. This newsletter will be published quarterly in March, June, September, and December.
Carrie Foss and Carol Ramsay have updated the Web site for the 2002-2003 Pesticide Education Training season. The Web site has dates, locations, agendas, and registration forms. Point your Internet browser toand select "Recertification and Pre-License Training." Each licensed pesticide applicator in Washington State should receive a hard copy of this information in the mail by the second week in October. Printed copies of the information are also available through county Cooperative Extension offices. For those waiting to hear from WSDA regarding their recertification status, those reports should be in the mail in early November.
Washington State University (WSU) provides pre-license and recertification training for pesticide applicators. Pre-license training provides information useful in taking the licensing exam. Recertification (continuing education) is one of two methods to maintain licensing. (The other is retesting every five years.)
Course registration (including study materials) is $35 per day if postmarked 14 days prior to the first day of the program you will be attending. Otherwise, registration is $50 per day. These fees do not include Washington State Department of Agriculture (WSDA) licence fees. For WSDA testing sites, schedule, or other testing information, call 1-877-301-4555.For more detailed information about WSU's pesticide applicator training, call the Pesticide Education Program at (509) 335-2830 or visit the Web site.
Mosquito Meeting to Address West Nile Virus
The American Mosquito Control Association (AMCA) will hold its annual meeting on March 2-6, 2002, in Minneapolis, Minnesota. The agenda will include major sessions on West Nile Virus. For more information on this meeting and other mosquito control topics, including a searchable database on West Nile Virus information, see the AMCA Website |
 |
Go to this issue's Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page