


To see this report in Portable Document Format (PDF), please
click on the button. ![]() Should
you not have Adobe Acrobat Reader (required to read PDF files),
this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Should
you not have Adobe Acrobat Reader (required to read PDF files),
this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Open Forum: In an attempt to promote free and open discussion of issues, The Agrichemical and Environmental News encourages letters and articles with differing views. To discuss submission of an article, please contact Dr. Allan Felsot at 509-372-7365 or afelsot@tricity.wsu.edu; Dr. Catherine Daniels at 509-372-7495 or cdaniels@tricity.wsu.edu; or Dr. Carol Weisskopf at 509-372-7464. The newsletter is available in a hardcopy version for a $15 yearly subscription fee. Please contact newsletter editor Sally O'Neal Coates at 509-372-7378 or scoates@tricity.wsu.edu for details.
Return to Table of
Contents for the December 1998 issue
Dr. Doug Walsh, Agrichemical & Environmental Education Specialist, WSU
On October 8, 1998, Steve Appel, President of the Washington State Farm Bureau, sent a letter to James Zuiches, Dean of the College of Agriculture and Home Economics at Washington State University. The letter expressed concerns regarding potential regulatory actions that may stem from implementation of the Food Quality Protection Act of 1996 (FQPA) and the potential fallout for agricultural producers in Washington. Specifically, Appel asked if data had been compiled on the economic impact of banning organophosphate and carbamate insecticides. Additionally, he asked WSU to respond to seven detailed questions.
Dean Zuiches appointed Associate Dean Jim Carlson to formulate a response to these questions. On October 12, Jim Carlson assembled a group of WSU faculty with relative expertise in these broad topics, including Allan Felsot, Catherine Daniels, and me, from the Food and Environmental Quality Laboratory. I was asked to respond to Question 4. Following is an excerpt of my reply as well as some additional comments.
It is obvious that substantial economic and pest control risks and costs would result from a ban on key organophosphate and carbamate pesticides. At worst, a total ban of organophosphates and carbamates could result in complete crop failures from damage caused by insect and mite pests for which there are no viable chemical, cultural, or biological alternatives.
Biopesticides and softer chemistry pesticides are often listed as alternatives to organophosphates and carbamates. They can be more costly and less effective at suppressing pest insects and mites. (If biopesticides could provide consistent and cost-effective pest control, why don't they command a greater part of the pesticide market already?)
Some biopesticides and cultural techniques, including mating disruption, contribute to the economic control of some pests. Several new biopesticide chemistries provide extremely effective control of some key pests, but in a trend that follows traditional chemistries, development and registration for these products has lagged on minor crops. Other concerns associated with biopesticides are that they have often proven ineffective at suppressing arthropod pests with piercing-sucking mouthparts like spider mites, aphids, and true bugs, and pests with rasping sucking mouthparts like thrips.
Biopesticides often target species or specific groups of insects. Organophosphate and carbamate insecticides will typically control a broad range of pest insects and offer some flexibility in the length of residual control provided since some organophosphates and carbamates have short residual periods while others can remain biologically active for an extended time period.
Pests can often be controlled biologically if disease or beneficial predators or parisitoids are sufficiently abundant. Numerous incidences of introduction of exotic beneficial organisms resulting in successful suppression of pests have been documented. There are several citations of successful pest control resulting from inundative release of beneficial arthropods. However, cost of these biological methods can be prohibitive. The biological control agent may be expensive and since the organisms are alive they must be distributed into the field gently. Releases can entail some risk-taking since the quality and quantity of the biological control purchased can vary between vendors and between orders from the same vendor. Release of beneficials requires patience since there is an establishment period and typically no rapid knock down of infesting pest populations. It can also be labor- and/or time-intensive since the pest population must be sampled and monitored closely so that it doesn't exceed the control-action threshold and require (typically chemical) suppression. Inundative releases have focused primarily on high-value/high-management crops or instances where regulatory or pest resistance constraints have left producers with few other effective alternatives.
Use of photo-stable synthetic-pyrethroid insecticides would increase if organophosphate and carbamate insecticides were banned. I have been involved with research proving that pyrethroid insecticide residues persist at biologically disruptive concentrations for months and possibly even years following field application on plant materials. Pyrethroid residues accumulate and are additive from multiple applications. Pyrethroids are biologically disruptive and their use will often result in a resurgence of the target pest or lead to an outbreak of secondary pests including spider mites or thrips. Additionally, many key pest species have documented histories of rapidly developing resistance when exposed to multiple pyrethroid applications in a season or over several years. Agricultural producers could be faced with increased costs associated with pest control of secondary pests and crop damage caused by secondary pests. Pesticides registered for control of secondary pests can have strict regulations regarding use and re-entry. In many minor crops timing of cultural manipulations to the crop is critical to production. Producers will incur economic loss from the downtime associated with long re-entry intervals if they are not able to work in their fields at critical crop production junctures.
A ban of organophosphate and carbamate insecticides could change the arthropod complex in the agroecosystems of many minor crops. There's no telling what pest currently suppressed by organophosphate and carbamate insecticide technologies could emerge from a complex in which it was no longer suppressed.
Additionally, it could prove difficult to eradicate or suppress exotic pests that might be introduced into Washington in the future without organophosphate or carbamate pesticides. The establishment of new exotic pests in Washington could result in significant economic losses if agricultural commodities are banned from domestic or export markets. It is nearly impossible to estimate the effect of the introduction of an exotic pest on the agricultural community.
Agricultural producers have relied on organophosphate and carbamate pesticides to maintain effective pest control; this reliance has mitigated risk to farm loan and crop insurance underwriters. The uncertainty associated with a ban on organophosphates and carbamates would undoubtedly increase crop insurance and farm loan interest rates, thereby increasing producer's costs in yet another way. Additionally, a credit crunch could result as loan underwriters became unwilling to accept the increased risk.
Crop producers using marginally effective pest controls would not only incur costs due to crop damage but would also be faced with increased labor costs for culling damaged product. Chemical alternatives could create more problems than they solve. Future ecological considerations are a wild card with potentially disastrous consequences. Increased difficulty in producing a salable crop economically could result in increased insurance and financing costs, or even loss of insurability or credit.
Dr. Doug Walsh is the Agrichemical & Environmental Education Specialist at WSU's Irrigated Agriculture Research & Extension Center in Prosser. He can be reached at (509) 786-9287 or dwalsh@tricity.wsu.edu. Answers to the other six questions posed by WSFB will be summarized in the January issue of AENews.
Dr. Allen L. Jennings, director of USDA's Office of Pest Management Policy
The Food Quality Protection Act of 1996 (FQPA) charges the Department of Agriculture (USDA) with creating a minor use program, furthering integrated pest management (IPM) research and application and gathering basic data used in pesticide exposure analysis. These data are fundamental components of the Environmental Protection Agency's (EPA's) risk assessments and include pesticide use surveys, pesticide residue analyses, and food consumption surveys.
The Vice President's April 8, 1998, memo to USDA Secretary Dan Glickman and EPA Administrator Carol Browner emphasizes the role of USDA in its partnership with EPA in FQPA implementation. USDA is committed to a close, long-term working relationship with EPA to help ensure that FQPA implementation is based on sound science, transparent processes, ongoing stakeholder involvement, and, when necessary, orderly and predictable transitions in the pest management strategies of agricultural producers.
USDA will help ensure that pesticide risk assessments are based on sound science by continuing to provide high quality and credible data exposure assessments. The department will seek to expand the current data collection programs and utilize the expertise of the land grant university system to meet the demands of FQPA. USDA will maintain ongoing stewardship of the data by clearly identifying its strengths, weaknesses, and limitations, and will work with EPA to ensure appropriate and optimal use of the data.
Specific data collection efforts include surveys of pesticide use and integrated pest management practices, food consumption surveys, and pesticide residue monitoring. The department will work with EPA to identify and develop improved risk assessment tools and will be an active partner with EPA in the development of chemical risk assessments and the underlying risk assessment policies and guidelines.
When risk assessments, based on the best possible science and data, indicate excessive risk, USDA will use the crop-pest profiles now under development to identify crop production issues, pest management alternatives, research needs, and opportunities for risk mitigation. The department will ensure that agricultural producers are aware of and involved in the risk management processes. In consultation with crop production experts and agricultural producers, USDA will effectively reduce risk to acceptable levels while preserving critical pesticide uses, particularly Integrated Pest Management (IPM) and resistance-management programs. Through the Office of Pest Management Policy, the land grant institutions have begun the process of developing and publishing (on the Internet) state-level crop profiles. These profiles summarize basic agronomic information on each crop in each state and focus on major pests and management practices. Specific attention is paid to IPM and resistance management programs and needs.
In those cases where cancellation of a critical use is the only effective regulatory mechanism to reduce risk to acceptable levels, USDA will work with EPA, agricultural producers, and crop production experts to develop and implement approaches that allow growers to move to new or revised pest management systems without significant disruption of domestic production.
Because the successful introduction of alternative pest management controls depends, in part, on the timely registration of new products, USDA will work closely with EPA to develop priorities and schedules for decision making. Crop profiles will provide much of the basic data needed for determining priorities and identifying vulnerable crops. As such, the crop profiles are basic to both risk management and to the development of transition strategies for crop-specific pest management needs.
USDA has also developed a "pipeline database." The database identifies unregistered products that are likely effective pest management chemicals for specific crop-pest combinations. The database consists of research currently under way, products that have been in use under an emergency exemption, and products currently being used under experimental use permits pending full registration applications. Products in any one of these categories have the potential for registration within a few years and could be key elements in strategies to phase out or reduce the risk of particularly high-risk pesticide uses.
Finally, both short- and long-term pest management research programs are being examined and retooled to respond to FQPA-driven needs. Processes for establishing research priorities and programs will make much greater use of stakeholder input.
For questions regarding crop profiles, contact your state's Pesticide Impact Assessment Program (PIAP) liaison, or Dr. Catherine Daniels, WSU Pesticide Information Coordinator and Washington state PIAP liaison, cdaniels@tricity.wsu.edu or (509) 372-7495.
Jane M. Thomas, Pesticide Notification Network Coordinator, WSU
When the Environmental Protection Agency (EPA) announced early this year that they were proposing to revoke 871 pesticide tolerances, our keenly intelligent initial response was "They're gonna do what?" followed quickly by "Cancel 871 tolerances??!! Is this a typo?" What was going on? Was EPA undertaking some sort of tolerance deforestation effort? Visions of tolerances toppling right, left, and center ran rampant. Besides noting the sheer number of tolerances being discussed, alert Federal Register reviewers like us also noticed the mention of phosphamidon use on apples. Alarm bells very disharmoniously began to sound: Caution! Caution! Caution!
Tolerance reassessment requirements set forth in the Federal Food, Drug, and Cosmetics Act task EPA with reviewing some 3,200 tolerances by August 1999. In efforts to meet this mandate, EPA reviewed their books and identified tolerances they believed met two criteria: first, that no current registrations existed for these specific crop-pesticide combinations, and second, that existing stocks of products labeled for these uses had been depleted. This proposed action was basically a "housecleaning" effort by EPA, intended to delete any unnecessary tolerances being carried in the regulations. It has been the agency's policy to issue a final rule revoking tolerances when there are no active registrations under FIFRA (Federal Insecticide, Fungicide, & Rodenticide Act). EPA's concern has been that retaining "unnecessary" tolerances might encourage the misuse of pesticides. With this in mind, perhaps our concern was unfounded. Or was it...?
Our initial action (and, yes, probably the easy part) here at WSU's Pesticide Information Center (PIC) was a search of the Pesticide Information Center On-Line (PICOL) label database based on each proposed revocation. We entered each crop-pesticide combination into the database to see if PICOL showed any active registrations. Next, as all good PICOL users must, we pulled the labels and had a look-see to verify that, where the database said they existed, registrations really were still in place. We found many cases where registrations still existed in Washington for crop-pesticide combinations EPA was proposing to axe.
At this point it became clear to the PIC staff that it was time to bring in reinforcements and, in a transparently harmonious manner, the Washington State Department of Agriculture entered the fray. WSDA's Pesticide Management Division agreed to act as the interface with EPA. They kindly stepped up and took the lead in collecting more registration data, contacting growers and commodity/commission groups, and then submitting comments on behalf of Washington agriculture to EPA. When the dust settled, most of the tolerances on which WSDA commented were left standing.
Here's a quick look at what WSDA accomplished:
EPA proposed revoking the tolerance for use of phosphamidon on apples, believing that existing stocks had been used. WSDA was able to point out that while no active phosphamidon registration existed, Washington apple growers had retained a sizable phosphamidon inventory-a six-to-eight-year supply, according to industry representatives' estimates. In their October 26, 1998, Federal Register notice, EPA agreed not to revoke the tolerance for phosphamidon on apples in this action. But EPA does intend to revoke this tolerance so if you have phosphamidon on hand, use it up. (Note that Northwest Wholesale, Inc. also submitted comments on this proposed revocation, stating that they believed existing stocks might last up to 10 years.)
WSDA commented that active registrations existed for the use of cryolite on these crops. The collard use is via a Section 3 label and the other uses were, at the time, authorized via SLN WA-980001 (Special Local Needs, derived from Section 24c of FIFRA). EPA acknowledged that including collards in the proposed revocations was an error. (It is noteworthy that out of all the registrants who must have reviewed this notice, WSDA was the only commentor regarding the cryolite-collard tolerance.) Following the February 5 initial notice, EPA notified WSDA that blackberries, boysenberries, dewberries, loganberries, and youngberries must be removed from the 24c label; on May 13, 1998, WSDA issued a revision to SLN WA-980001 limiting its use to blueberries, raspberries, and strawberries. As an aside, WSDA indicated that Gowan would request these crops be reinstated on the 24c after IR-4 submits completed data packages. (In an effort toward greater transparency, why not retain the tolerance so that it will be in place when the 24c is revised again?)
EPA proposed revoking the exemption from the requirement for a tolerance for copper oleate on all crops. WSDA, along with Griffin Corp., requested that the exemption be retained and EPA's 10/26/98 decision was not to revoke the exemption at this time.
WSDA verified that active registrations exist for these uses and requested that EPA retain these tolerances. EPA's response was as follows:
Since ODDA is a lepidopteran pheromone, it will remain covered under the broader tolerance exemption of 40 CFR 180.1153 Lepidopteran pheromones; exemption from the requirement of a tolerance. Therefore, the current tolerance exemptions listed for ODDA under 40 CFR 180.105 are not needed and will be revoked by the Agency.
WSDA pointed out that a 24c registration existed (WA-940029) for the use of ferbam on boysenberries. EPA's decision was not to revoke the tolerance on boysenberries. As a comment, this seems only appropriate (not to mention transparent and harmonious), since one of the criteria for retaining tolerances is that they have a FIFRA-registered use and SLNs clearly meet this condition.
WSDA found that trichlorfon was registered for use as a pour-on insecticide for cattle. They, along with Bayer, requested EPA retain the tolerances for this compound in cattle fat, meat, and meat by-products. EPA has agreed not to revoke these tolerances.
Both Monsanto and WSDA commented that active registrations still existed for propachlor on corn. EPA agreed that the proposed revocation for tolerances for propachlor on corn forage and grain was in error and agreed to retain these tolerances.
WSDA was joined by the Canadian Horticultural Council, Amvac, and Valent in commenting on proposed revocations for a host of naled tolerances. WSDA's comments were limited to cucumber and legume uses where active registrations still exist in Washington. The final decision was in favor of retaining both the cucumber and forage legume tolerances.
WSDA identified active registrations for use of atrazine on grass. EPA acknowledged that Drexel has registrations for use of atrazine on orchardgrass, pastures, and rangeland. EPA's final decision was to retain the atrazine tolerances on range grass, orchardgrass, and orchardgrass hay.
Uniroyal commented that it had a product labeled for use on cherries and, while it was supporting both sweet and tart cherry tolerances, it would not support use on other stone fruit. (In its comment letter to EPA, WSDA also requested that the dichlobenil-stone fruit tolerance be retained; however, in the final Federal Register notice, EPA did not mention WSDA's comments.) EPA determined that it would not revoke the stone fruit tolerance for dichlobenil until it had reviewed existing data on cherries and established an appropriate tolerance level. At that time, EPA intends to proceed with revocation of the stone fruit tolerance.
With more tolerance reviews ahead between now and August 1999, a review of the "lessons learned" from this process is in order. In many cases, EPA was proposing to revoke tolerances where active registrations, either Section 3s or 24cs, existed. In other cases, EPA was proposing to revoke tolerances where the use had only recently been deleted from the product label and substantial product was still in trade channels. Therefore, the first lesson learned is review proposed revocations with respect to your own crop-pesticide combinations-it's time well spent.
Lesson number two is take the time to submit comments to EPA. The tolerance you save may be your own. Of the twenty-two ingredients for which EPA proposed tolerance or exemption revocations, WSDA submitted comments that were included in decisions made on ten of the ingredients. Of the ten issues on which WSDA submitted comments, eight were decided favorably, one was found to be a non-problem, and one (cryolite on berries) was decided negatively. Based on the Federal Register notice announcing EPA's final decision, it is interesting to note that WSDA submitted a greater number of comments than any other party involved in this action. The other commentors were twelve registrants, two international concerns, and seven research/commodity groups. No other state's department of agriculture submitted comments on this action.
And our point is? Hats off to WSDA for a great effort!
Jane M. Thomas is the Pesticide Notification Network (PNN) Coordinator for the Pesticide Information Center (PIC) at WSU. She can be harmoniously contacted at (509) 372-7493 or transparently and electronically accessed at jmthomas@tricity.wsu.edu.
A suggestion was recently made that anyone associated with Food Quality Protection Act issues be fined $5 each time they use a derivative of buzzwords du jour "transparent" or "harmony" in any written or oral communication. In an effort to live in harmony with this concept the author has donated $45 to the Pesticide Professionals Entertainment Fund (PPEF).
What--you have a better idea? Talk to Dear Aggie: dearaggy@tricity.wsu.edu.
Would you like to receive a hard-copy subscription of Agrichemical & Environmental News? If so, simply send a $15 check, made out to WSU, to:
Pesticide Information Center WSU Tri-Cities 2710 University Drive Richland WA, 99352-1671Include the name and address to which you would like the subscription mailed. New subscribers whose check is received by December 15, 1998, will receive a 12-month subscription beginning with the January issue. Current subscribers also need to get checks to us by December 15, 1998, to receive their subscription uninterrupted. The $15 annual fee, which covers the costs of printing and mailing the newsletter, gets you 12 issues of fascinating information and riveting reading.Web access remains free. If your check arrives after December 15, we will make all efforts to include you in the January mailing, but if you're late we will only guarantee that you will begin 1999 with the February issue. If you have any questions or comments please direct them to Sally O'Neal Coates at (509) 372-7378, or email scoates@tricity.wsu.edu.
Here we go again. Agriculture, having survived the heavy metal mania of last year, is now bracing for the dioxin dilemma. During the process of testing fertilizers for heavy metal content, the Washington Department of Ecology discovered that some fertilizers and amendments contain dioxins. Now WDOE has proposed a study that would sample agricultural soils from around the state for analysis of dioxin levels. Meanwhile, WDOE released a report in July, the Washington State Dioxin Source Assessment (Yake et al. 1998), that details sources of dioxin generation and estimates loads emitted to the environment. Pertinently, some of these dioxin generators are sources for agricultural amendments. For example, hog-fuel (wood-waste) boilers create an ash that can be used as a liming amendment.
Given the ubiquitous nature of dioxin sources in the state, and the fact that amendments contain dioxins, a hypothesis that agricultural soils are contaminated with dioxins seems plausible. But can it be proven that fertilization practices have significantly altered levels of dioxins in soil? More importantly, if dioxins are found in soil, do they pose a hazard by significant transfer to food?
The term dioxins actually represents 210 different compounds. The basic structure of dioxins, shown in the figures, consists of two six-membered carbon rings (i.e., benzene rings) joined together by either one or two oxygen atoms. The compounds with two oxygen atoms bridging the benzene rings are called dibenzodioxins (or dioxins), and those with one oxygen atom are called dibenzofurans (furans). Because both types of basic structures have one or more chlorines attached at different positions on the benzene rings, they are properly called polychlorinated dibenzodioxins (PCDDs) and polychlorinated dibenzofurans (PDCFs). The compounds of concern are those with four or more chlorines. In this essay, the term dioxin will be used as a convenient designation for both PCDDs and PDCFs.
Each of the carbon atoms of the benzene rings is assigned a number as shown in the figures. Thus, 2,3,7,8-tetrachlorodibenzodioxin (commonly abbreviated as TCDD) has four chlorine atoms attached to the carbons of the benzene rings at the positions indicated by the numbers. Given the number of available carbon atom positions accommodating four or more chlorine atoms, 75 and 135 forms of PCDDs and TCDFs, respectively, are possible. Each form is called a congener.
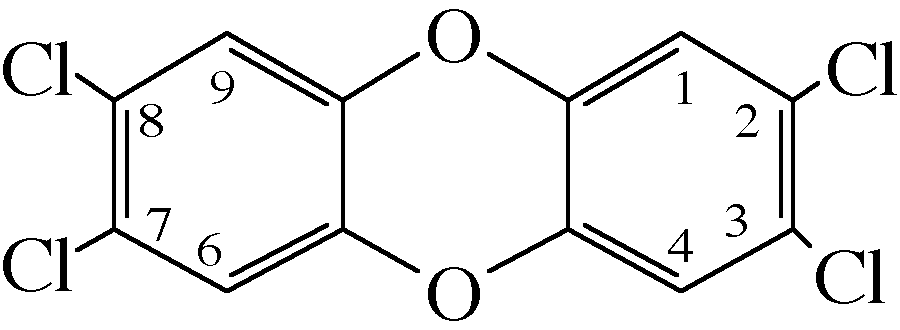
Structure of 2,3,7,8-tetrachlorodibenzodioxin. The carbon atoms are not shown, but are represented by the points of each hexagonal benzene ring. There are 75 possible forms having one or more chlorines.
Structure of 2,3,7,8- tetrachlorodibenzofuran. There are 135 possible forms having one or more chlorines.
Among the dioxin congeners, TCDD is considered the most toxic. TCDD was first discovered over thirty years ago as an impurity in pesticide formulations containing the herbicide 2,4,5-T, the wood preservative pentachlorphenol, and antibacterial soaps with the active ingredient hexachlorophene. Alarm swept through the toxicological community because TCDD had an LD50 (lethal dose to 50% of the test animals) to guinea pigs of one microgram per kilogram of body weight (1 µg/kg) and to rats of 22 µg/kg! (Consider that aldicarb (Temik) is one of the most toxic insecticides, and its LD50 is 1000 µg/kg in rats.) Given the presence of TCDD in commonly used products, toxicologists were relieved to find that humans seem to be thousands of times less susceptible to TCDD than rodents. Indeed, the only known acute effect of TCDD exposure in humans is a severe acne-like skin disease called chloracne.
EPA and WHO (the United Nations World Health Organization) now consider dioxin a human carcinogen, largely based on the weight of the evidence from rodent feeding studies and epidemiological studies. One cancer in particular, soft-tissue sarcoma, has been linked with exposure to TCDD, but it is a very rare disease. While the carcinogenicity of TCDD and other dioxins is still arguable in the toxicological community, the debate has moved beyond cancer to endocrine-disrupting effects. Indeed, high doses of TCDD given at appropriate times during a rat's pregnancy can cause birth defects and reproductive abnormalities in the offspring. Throw in possible effects on male testosterone levels, decreased sperm counts in rats, and altered immune systems, and TCDD would be classified as an endocrine disrupter.
EPA in 1994 released a draft reassessment of dioxin risks. EPA's Scientific Advisory Board reviews such drafts. Owing to conflicts over some of the findings in the report, the EPA has yet to release its final draft. Editorial essays in scientific journals highlight the conflicts over the conclusion drawn in the draft document (Clapp et al. 1995; Environ Dioxin Risk Characterization Expert Panel 1995). Environmental epidemiology applied to chemical exposures is always open to negative criticism, but why so much delay about chemicals that are not intentionally manufactured and serve no useful purpose?
EPA's final conclusions about dioxin risk will determine the course of regulation over dioxin emissions. But it is easier to make regulations than to carry them out, because PCDDs and PCDFs are naturally occurring compounds that apparently have been in the environment since the first fire on earth. In the late 1970s, it was discovered that dioxin is produced in combustion processes such as waste incineration. While some blame PVC plastics and packaging materials for production of dioxin during incineration, we now know that dioxin is produced whenever wood or other naturally occurring fuels including coal are burned. Indeed, WDOE found that hog fuel boilers are an important source of dioxin emissions in Washington. Furthermore, volcanoes and forest fires reportedly release dioxin (Gribble 1994, Takizawa, et al. 1994).
Scientists have drilled cores out of lake bottoms. The age of the sediments is easily determined. Analysis of these sediments for dioxin has definitively proven that all the congeners of concern were present in the environment during the earliest years of industrialization (Juttner, et al. 1997). Nevertheless, dioxin levels started to increase around 1935 and trended sharply upward after World War II (Brzuzy and Hites 1996). Thus, humans may have always been exposed to dioxin, but are we being exposed to too much dioxin now? The answer to this question necessitates an inventory of dioxin sources and sinks, and a calculation of expected concentrations relative to estimated tolerable daily intakes.
The analytical chemistry of dioxin is one of the most demanding types of environmental analyses. Not only are the concentrations incredibly small, expressed in parts per trillion (ppt), but 136 possible congeners of PCDDs and PCDFs have four or more chlorines. Biochemical toxicology studies have shown, however, that only compounds with chlorine in the 2,3,7,8 positions are toxicologically relevant. Therefore, only 17 congeners are routinely analyzed. But analysis of this many compounds at one time requires exhaustive steps be taken to avoid confusing such small amounts of these compounds with other naturally occurring compounds.
Another problem is how to express the amount of all 17 compounds in a single sample. If each of the 17 compounds were of equal toxicity and caused ill effects by a common mechanism of toxic action, then they could simply be added together. But the most toxic congener is 2,3,7,8-TCDD followed by 1,2,3,7,8-pentachlorodibenzodioxin (PeCDD), which is nearly as toxic in guinea pigs, mice, and chicks. The other congeners are ten to hundreds of times less toxic.
In finding that dioxin interacted with a specific cell protein called the Ah receptor to initiate a cascade of biochemical reactions, a method for dealing with the aggregate total of the 17 toxic dioxin congers was born. Studies showed that each of the congeners had its own characteristic affinity for binding to the Ah receptor, with TCDD having the highest affinity (Safe 1990). Furthermore, none of the toxic congeners were metabolized nor eliminated from the body except over very long time periods. Indeed, a lot of the body burden of dioxin is stored in fat (adipose tissue), and tiny amounts slowly diffuse into the blood. Thus, because each of the congeners is very stable in the body, the toxic hazard of any one of the congeners could be expressed relative to TCDD as a toxic equivalency factor (TEF). Thus, TCDD has a TEF of 1, PeCDD has a TEF of 0.5, and the least toxic congener, octachlorodibenzodioxin (OCDD, 8 chlorines), has a TEF of 0.001.
To express the aggregate concentration of the toxic congeners, soil, water, or tissues are analyzed for each of the 17 toxic congeners. The results are usually expressed as picograms per gram of material (pg/g), which is identical to a nanogram per kilogram (ng/kg) or a ppt. The concentration of each congener is multiplied by its characteristic TEF resulting in a number called the TEQ (toxic equivalence). All the TEQs are then added together to yield the sum TEQ. Thus, when any report expresses the concentrations of the toxic PCDD and PCDF congeners, it is really expressing the TEQ (as pg/g or ng/kg) relative to TCDD.
Extensive sampling and analysis for dioxins have shown that the absolute concentrations of the comparatively higher chlorinated congeners can be thousands of times greater than TCDD. However, changing the concentration to a TEQ also changes the perspective to one of lesser hazard. For example, in a nationwide study of dioxin in municipal sewage sludges, EPA reported that the median concentration of OCDD was 3500 ng/kg, which translates to a TEQ of 3.5, nearly the same as the TCDD median concentration of 4.4 ng/kg (Jones and Sewart 1997).
One flaw in the TEQ system persists. The TEQ concept was developed to consider a mixture of the 2,3,7,8-substituted PCDDs and PCDFs already in a biological matrix. While the congeners could be described as having similar physicochemical properties (e.g., very low water solubility, low vapor pressure, and a very high tendency to sorb to soil), their transfer rate from soil or plants to organisms can differ significantly (Jones and Sewart 1997). These discrepancies could result in overestimation of the real hazard of the more heavily chlorinated congeners. Despite this glaring problem, the universally accepted practice is to express concentrations of dioxin as TEQs.
Dioxin is emitted from two sources, combustion and chemical manufacturing. The consensus of environmental chemists today is that combustion sources emit nearly all the dioxins. Incidental chemical manufacturing processes, for example synthesis of chlorinated chemicals or paper bleaching, account for very little of the estimated global mass balance of dioxins (Brzuzy and Hites 1996). Municipal waste incineration accounts for over 30% of the global mass balance. Pertinently, the data shown in Table 1 relied upon a soil collection technique, indicating that soil may be the most important sink for dioxin deposition.
| SOURCE | KILOGRAMS/YEAR |
| Municipal Waste Incineration | 1130 |
| Biomass Combustion | 350 |
| Iron Metals Production | 350 |
| Cement Kilns (burning hazardous waste) | 680 |
| Cement Kilns (no hazardous waste) | 320 |
| Secondary Copper Smelting | 78 |
| Medical Waste Incineration | 84 |
| Unleaded Fuel Combustion | 1 |
| Leaded Fuel Combustion | 11 |
| TOTAL | 3004 |
Another little studied source of dioxin is municipal sewage sludges (Jones and Sewart 1997). Dioxin may get into sludges from wastewater containing combustion-derived dioxin, from road run-off, from chemical manufacturing, or from impurities in paper products, detergents, and dyestuffs. Dioxin has been hypothesized to form during chlorination of tap water or during wastewater treatment. Because of dioxin's propensity for sorption to solids, municipal wastewater treatment would essentially filter out the compounds into the sludge.
Humans are exposed to dioxin almost solely through the food chain. The lead hypothesis for explaining exposure is that it occurs through the atmospheric deposition of combustion products on foliage eaten by livestock. Because dioxin is very stable and resistant to biodegradation, it will be stored in meat and milk and passed to consumers. Nearly 70% of total dietary dioxin exposure has been estimated to come from meat and milk products in European diets (Jones and Sewart 1997). Fish will also bioaccumulate dioxin, becoming a major source of exposure for cultural groups relying heavily on its consumption.
Direct consumption of vegetables and fruit containing dioxin residues from atmospheric deposition or from soil particle contamination (which largely affects root crops), probably accounts for no more than 10% of the estimated dietary intake (Jones and Sewart 1997).
The background concentration of TCDD in humans is considered 79 ppt (pg/g fat) (Fries and Paustenbach 1990, Anonymous 1995). According to one exposure analysis, to maintain the TCDD background concentration (i.e., a steady state concentration), a human would have to be exposed to 0.41 pg TCDD per kg body weight per day (pg/kg/d). Vegetables only contributed about 1% to the amount of TCDD to which a person is exposed each day to maintain the current background body burden. Furthermore, beef and milk contributed no more than 20% of the daily intake needed to maintain the background burden. Thus, although food is nearly the sole source of dioxin exposure, it contributes little to the background body burden (Fries and Paustenbach 1990).
Little monitoring of fertilizers and soil amendments has been reported so any definitive answers await further study. WDOE analyzed several soil amendments and reported finding various levels of dioxin TEQ (as ppt) ranging from < 1 ppt in cement kiln dust to 340 ppt in granular zinc fertilizer made from steel mill flue dust (Washington Department of Ecology 1998). When scaled up to the expected rates of amendments application, the soil concentrations would not have changed relative to expected background levels. In England, these background levels have been reported to range from 223 ppt, with urban soils being on the high end of the range (Jones and Sewart 1997).
Sewage sludge has been legally applied as a fertilizer to U.S. and European soils for many years. A survey of different municipal sewage sludges in the U.S. indicates that the average TEQ is 83 ppt with a range of 0.52321 ppt. In England, the average sewage sludge had 72 ppt TEQ, but the range was only 19206. Researchers estimated that atmospheric deposition of dioxin exceeded inputs from sewage sludge by a factor of 10. Thus, even when amending soil with a seemingly high source of dioxin, the overall contribution to the soil load is practically nil.
Several studies show that even where dioxin is deposited in soil, uptake by crops is negligible. One reason is that dioxins are bound strongly to soil and show almost no capability for transfer from the soil to the root and from the root into the stem. Lettuce, peas, and hay did not absorb dioxin from soil even at contamination rates up to 6000 ppt TEQ (Hulster 1993, Muller et al. 1994). Only root crops such as potato and carrot had evidence of dioxin absorption, but it was limited to the peel and easily removed. As hypothesized, airborne deposition was the source of crop contamination.
One interesting exception to the potential for root uptake of dioxins occurs with two plants in the Cucur-bitaceae family, zucchini and pumpkin. These plants seem to exude chemicals that might make the dioxin more bioavailable for root uptake (Hulster et al. 1994). Even so, the cucumbers studied were contaminated by airborne deposition rather than from soil uptake.
Dioxins, a term commonly used to represent at least 17 toxic compounds, are ubiquitous in the environment. They are naturally produced in all combustion processes whether chlorine-containing plastic materials are present or not. Dioxins are found in lake sediments hundreds of years old, but concentrations rapidly increased after World War II. Combustion sources are by far the major source of environmental distribution of dioxins, atmospheric deposition is by far the most common exposure route, and humans are exposed via food.
Some good news looms on the horizon. A recent trend analysis indicates that atmospheric emissions may be dropping, with consequent decreases of dioxin in food (Alcock and Jones 1996). Chemicals that had contained dioxin as a result of incidental contamination during manufacturing are increasingly banned, further reducing the importance of this pathway of exposure.
Over a billion dollars has been spent worldwide studying dioxin. We already know a lot about its environmental chemistry, and estimates of our daily exposure are being determined with more confidence than a decade ago. A preponderance of studies show that crops are contaminated largely because of combustion processes, not because of soil residues created by fertilizer or soil amendments. WDOE may make a good case on academic grounds that it would be interesting to know how much dioxin is in our soils. However, it seems unlikely they will unearth any dirty little secrets.
Dr. Allan Felsot is an Environmental Toxicologist at WSU. He can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
Is produce marketed as having "no pesticides detected" actually free of pesticides? Are organic fruits and vegetables free of pesticides? Would an increase in the number of commodities in which pesticides were detected indicate that our food supply was becoming increasingly contaminated? If you were going to buy a used car, and the dealership told you that they found no defects in their examination, would you look under the hood anyway?
Most people have a much better idea of the workings of an automobile than of pesticide residue chemistry. At least they know there should be an engine in there somewhere, and that the usual number of wheels is four. In evaluating our fruits and vegetables before we buy or eat them, we're not so self-reliant-not even chemists have a gas chromatograph in the garage to test the pesticide content claims of the local supermarket. Fortunately, if we want to examine the pesticide content of our food supply, ample data are available.
The problem is not with data, but with its use and interpretation. A literature review illuminates an ever-growing problem with pesticide-related issues: pesticide detection limits are getting lower. What at first appears to be an advantage-better science resulting in lower detection limits-becomes a problem when the prevailing perception is that detections are, of themselves, related to safety. As detection limits get lower, instances of residue detection on crops increases. We are gradually approaching the analytical capability to detect pesticides in 100% of samples analyzed. The time has come to move toward food safety regulations based on concentrations of pesticides, not simple detection of them.
In-depth data has been compiled by the U.S. Food and Drug Administration (FDA, http://vm.cfsan.fda.gov) and by the California Department of Pesticide Registration (CDPR, http://www.cdpr.ca.gov). Both agencies monitor fruits and vegetables for violations of pesticide tolerances, "tolerance" being defined as the maximum pesticide concentration allowed in a commodity for which a registration exists. Detections of a pesticide on a crop for which there is no registration, and thus no tolerance, are also considered violations. Violations of either type can result in removal of the commodity from commerce. Because shipments from which samples are collected may be held until analyses are completed, these programs rely on rapid analysis methods with detection limits adequate for detection of pesticide concentrations in the vicinity of tolerances.
At the other end of the spectrum is the rigorous testing conducted by the U.S. Department of Agriculture Pesticide Data Program (PDP, http://www.ams.usda.gov/science/pdp/index.htm). The PDP is not under the time constraints of the FDA and CDPR surveillance sampling, as the PDP does not affect commodity distribution. The PDP strives for the lowest detection limits, which can require analysis times of weeks, rather than hours.
Summary data from FDA, CDPR, and PDP are presented in Table 1. The most recent available FDA data are from 1997, and for CDPR from 1995. PDP's most recent available data are from 1996. In each case, both imported and domestic fruits and vegetables were tested.
Program |
Commodity Types Sampled |
Number of Analytes |
Number of Samples |
Samples with Detections |
Samples over Tolerance |
Detections with No Tolerance |
| FDA | 70 | 366 | 7268 | 39% | 0.36% | 1.4% |
| PDP | 8 | 77 | 3618 | 74% | 0.22% | 4.5% |
| CDPR | 132 | 200 | 5502 | 35% | 0.31 | 1.35 |
As might be expected, the FDA and CDPR data show similar percentage instances of pesticide detections and tolerance violations. The PDP data, also unsurpri-singly, show a higher percentage of samples with detections and a higher percentage of detections of products for which no tolerance has been established.
If the number of pesticide detections were a direct measure of food safety, one could conclude that the samples analyzed by FDA and CDPR, with 35 and 39% detection rates, were twice as safe as those analyzed in the PDP, at 74% detections. In actuality, the difference is simply a demonstration of the impact of analytical methods with low detection limits.
A basic tenet of toxicology is that "the dose makes the poison." It is the concentration that determines exposure level, and thus impact, not the simple occurrence. Unfortunately, detections themselves are often used in comparisons of food safety regardless of concentrations, and are considered undesirable in and of themselves by the public and some regulators.
Which leads to the issue of detection of pesticides not registered for use on the commodity on which they are detected. In the PDP data, 4.5% of the samples contained such residues. PDP used twelve laboratories for their analyses. If the data were reassessed as if each pesticide/crop combination had been analyzed by the PDP lab with the least sensitive procedure, the total number of detections would drop only from 74 to 70%. But, the unregistered use detections would drop from 4.5 to 1.5%. If two thirds of such detections are eliminated using the worst detection limits, would apparent violations triple if the best were used?
It is long past time to disconnect detection capability from assumption of hazard.
When a chemist can find just about everything in just about anything, the concept of "pesticide-free" food or, for that matter "anything-free" food, becomes irrelevant. It might be useful simply to stipulate that we can detect at least one pesticide in any sample, and get on with the business of deciding what the data mean.
The tolerance should be considered as the touchstone for risk evaluation, with debate centering on the adequacy of existing and proposed tolerances.
But what of "unregistered use" violations-those for residues found on a crop for which there is no registration or label, and thus no tolerance? Such detections account for the majority of violations found. In these cases, it is simple detection, rather than concentration, that constitutes a violation. Again, the efforts should center on the establishment of risk criteria. One solution would be to expand the use of action limits.
DDT, although no longer registered in the United States, remains in the environment. DDT or its transformation products can still be detected at trace levels on commodities. Action limits have been established for DDT and other now-banned chlorinated insecticides, which function as de facto tolerances. Concentrations below these limits are considered acceptable, while those above result in the same market restrictions as over-tolerance findings. In the PDP data, 378 detections were of compounds in this class; none was found over the action limit.
Perhaps we should also stipulate that improving analytical capabilities will eventually result in detection of some level of unregistered agent on 100% of samples. Since this seems inevitable, we should deal with this issue before we run out of food that is legal to bring to market.
Despite the terminology, many "unregistered use" residues are the result of the physical and chemical behavior of pesticides rather than the intentional use of an unlabeled pesticide by a grower. Drift, crop rotations, packing conditions, post-harvest processing, and transport of pesticides in air, rain, fog, or dust can all give rise to low but detectable residues.
When I used to perform pesticide enforcement analyses, I enjoyed ferreting out concentrations of unlabeled pesticides at levels indicating an inappropriate application. However, when I detected pesticides at extremely low levels-concentrations easily attributable to environmental redistribution-this always presented an ethical, rather than analytical, dilemma. There is a difference, in intent as well as in concentration, between willful application of an unregistered pesticide and inadvertent receipt of a pesticide residue through fog or dust.
As detection limits improve, this dilemma will increase. Should a handful of wayward molecules be considered a violation? It is difficult producing good data for enforcement of regulations you believe are outdated. This has bothered me for 15 years, and we seem no closer to a solution.
"Inadvertent residue" is a much more accurate term than "unregistered use" for many detections. I first heard this term in the 1980s from my doctoral research advisor, Dr. Jim Seiber, in his studies of pesticide transport in fog. If we remove the connotation of will-fulness from these occurrences, perhaps they can be evaluated more rationally and regulated appropriately.
While the regulatory, chemical, and producer communities are in the throes of tolerance readjustment subsequent to enactment of the FQPA, it would be practical, efficient, and useful to at the same time establish action limits based on toxicological rather than analytical criteria, regulating unlabeled applications and allowing inadvertent residues. Pesticide chemists could then pursue the goals of better analysis methods, more sensitive laboratory equipment, and ever-decreasing detection limits without worrying about the impact on growers when dozens or hundreds of unregistered molecules are detected.
The final data set is from a study conducted by Consumers Union (CU), and described in the January 1998 issue of Consumer Reports. Although few details of the sampling and analyses are given, CU collected about 170 fresh produce samples from 4 commodities, and had them analyzed for over 300 pesticides.
Both the PDP and CU data included results from samples labeled as organic. In addition, the PDP samples contained a few for which claims of "no pesticides detected" had been made. (Such claims give no indication of the sensitivity of methods used or number of pesticides included in the analyses.) The CU samples also included a category they referred to as "green," which are commodities for which some claims were made of environmental friendliness in their production.
The number of samples from each category in the CU data is not given, but an even distribution among the three tested categories would be expected in a study designed to compare them. CU and PDP both reported pesticide detections and tolerance violations (Table 2). The most amusing part of these data is that the produce with "no detected residue" claims actually had a higher proportion of detections than any other category. Given the small number of samples with this claim, the difference is unlikely to be statistically significant.
Program |
Claim |
Number of Samples |
Number of Commodities |
Samples with Detections |
Samples over Tolerance |
Detections with No Tolerance |
| PDP | none | 3600 | 8 | 74% | 8 (0.16%) | 163 (4.5%) |
| PDP | organic | 10 | 4 | 0% | 0 | 0 |
| PDP | no residues | 6 | 3 | 83% | 0 | 0 |
| CU | none | 50-60 | 4 | 77% | 0 | 1 (2%) |
| CU | green | 50-60 | 4 | 55% | 0 | 0 |
| CU | organic | 50-60 | 4 | 25% | 1*(2%) | 0 |
| *concentration above criteria for organic produce eligibility, not above legislated tolerance | ||||||
The CU and PDP data are in close agreement (77 and 74%) on the proportion of detections in the samples for which no claims were made. On the other hand, the organic samples analyzed show quite a difference in detections, with CU finding residues in 25% and PDP having no detections. The limited number of organic PDP samples and the small number of commodities included in either data set may contribute to the difference, as might the increased number of pesticides screened in the CU analyses. Both data sets show considerably fewer detections in samples with organic labels, with an intermediate level of detections in the CU "green" samples.
One of the CU organic samples contained a pesticide concentration above that considered acceptable for designation as organic produce. One accepted criterion allows concentrations up to 5% of the established tolerance in commodities with the organic designation. Such criteria acknowledge inadvertent residues, rather than condoning use of synthetic pesticides by organic growers.
CU also generated a "toxicity score" for their report, using the pesticide identities and concentrations found. The CU report included the caution that botanical extracts and metallic compounds accepted in organic production can be as toxic as traditional pesticides. However, these alternatives to synthetic pesticides were not included in CU's chemical analyses, and so were not considered in generation of the "toxicity score". Produce with an organic label had lower computed overall toxicity, although omission of residue data for non-synthetic pest control agents makes the comparison moot. Although organic produce was designated as "least toxic," such terminology does nothing to improve my appetite.
We would do well to remember that organic farming is more than a method for delivering reduced-pesticide produce to the consumer, and detections are not the only measure of its value. It can also be an ethic, a philosophy, and a way of living in the environment, as well as a marketing tool.
Consumers want to buy safe produce. Through marketing, consumers have been educated to equate "no pesticide" with "safe." But claims of "no detected residue," while viewed by some as providing consumer information, may speak more to low-quality detection limits than high-quality comestibles. The PDP data indicate such a designation is considerably less predictive of pesticide detection than is the organic labeling, yet such claims may become commonplace when the FQPA-mandated brochure appears in supermarkets.
Even organic produce, while performing well by comparison to unlabeled and "no residues"-labeled produce in the PDP test, cannot promise "no pesticides." Wind, rain, fog, and dust can transport pesticides at levels sufficient to result in detections. And the rain, along with any trace levels of pesticides it may contain, falls equally on the organic and the non-organic grower. If the best CU can do is call organic samples the "least toxic," such unfortunate terminology proves a disservice to the organic and the non-organic grower alike, not to mention the consumer.
Analytical techniques are becoming more sophisticated and detection limits are decreasing in response to the need to provide more accurate risk assessments, deal with lowering tolerances, and prepare for the next generation of pesticide chemistries. Establishing tolerances, analyzing for tolerance compliance, and depending upon detection limits are only parts of the picture. Like an iceberg, much is below the surface.
Most of us sprinkle our gardens with water that meets the drinking water standards, but may contain pesticides at trace levels. If these pesticide levels were detectable, garden watering could be considered a tolerance violation. This is an example of why action limits should be instituted for inadvertent residues.
Although consumers may want to be able to buy produce without pesticides, it is impossible even for organic growers to deliver it. But the pesticide content of our food is well below levels considered acceptable. We have one of the most abundant, inexpensive, and safe food supplies in the world. There is no reason to dust off your gas chromatograph and test food before you eat it. But if your local supermarket starts to offer produce with "no detected residues," kick the tires before you buy.
Dr. Carol Weisskopf is an Environmental Chemist at WSU. She can be reached at (509) 372-7464 or at cpw@owt.com.
Cholinesterase testing can identify workers who, because of previous overexposure to organophosphates (OPs), are at increased risk of subsequent poisoning by these insecticides. Monitoring blood cholinesterase levels has traditionally involved drawing blood samples at a hospital or clinic and sending them to a laboratory for evaluation. Results can take as many as five days, during which time an at-risk worker may continue to be exposed.
In order to avoid such potentially dangerous delays, test kits designed to measure blood cholinesterase in the field have been developed. Unfortunately these kits have not been extensively tested in the field, and little is known about their performance compared to standard laboratory methods.
Dr. Matthew Keifer, co-director of the Pacific Northwest Agricultural Safety and Health Center, along with other researchers at the center, has been developing and evaluating procedures and methods for field testing blood cholinesterase levels. Part of this research has involved evaluating the accuracy of the EQM Testmate OP Kit. (Ed. Note: The EQM Testmate OP Kit was the only kit tested. This report is intended to provide general information, and is not intended as an endorsement for this particular brand of field-test kit.)
Cholinesterase, or more properly acetylcholinesterase, is an enzyme essential for normal functioning of the nervous system. In the body, acetylcholinesterase inactivates the chemical messenger acetylcholine, which is active at the junctions between nerves and muscles, between many nerves and glands, and at the synapses between certain nerves in the central nervous system.
Acetylcholinesterase is present in both red blood cells and plasma. The red blood cell acetylcholinesterate is also called rbc cholinesterase or erythrocyte cholinesterase and is commonly identified as AChE. The plasma cholinesterase, also called pseudocholinesterase or butyrylcholinesterase, as well as just cholinesterase, is commonly identified as PChE.
OP and carbamate insecticides are cholinesterase (ChE) inhibitors, affecting the enzyme's function both at the nerve endings and in the blood. When cholinesterase is inhibited significantly, the nervous system malfunctions. Affected individuals may exhibit pesticide-poisoning symptoms such as fatigue, lightheadedness, nausea, vomiting, and headaches. Severely low levels can result in death.
The activity level of cholinesterase is an indication of how much exposure to OP and carbamate insecticides workers have received.
The field-test kit evaluated by Dr. Keifer and his colleagues uses a light-emitting diode to measure the concentration of a chemical indicator that increases in proportion to the activity of cholinesterase in the sample being tested. Results are immediate and testing is simple and inexpensive. The kit can be used to analyze blood drawn from the vein or capillary blood obtained using a simple finger-stick. It can measure both PChE and AchE, and automatically corrects for hemoglobin levels that can affect the accuracy of the test.
The objective of the testing was to determine if a field-based kit, by providing immediate, on-site data, could facilitate more timely removal of overexposed workers, and if the kit was sufficiently accurate to be used as a substitute for lab testing. The three-year study involved eighty orchard workers in six eastern Washington orchards in the Yakima Valley and Tri-Cities areas who had regular exposure to Guthion, Diazinon and several other organophosphate and n-methyl carbamate insecticides.
The blood levels of the test group were compared to those of a control group of twelve workers with no known exposure. The results obtained using the field-test kit were compared to the findings of the Washington Department of Health (WDOH) laboratory for both PChE and AChE levels. Urine was also monitored for organophosphate breakdown products.
The first specimens were obtained about three weeks before the start of the spraying season to determine baseline cholinesterase levels. Three follow-up specimens were analyzed during the spraying season to determine if added exposure to the insecticides caused depressed cholinesterase levels. These tests were timed to coincide as closely as possible with pesticide applications in the participating orchards.
Analysis of urine samples from some of the workers confirmed that exposure was occurring, but no significant changes in ChE levels were noted using either the laboratory testing or the field-based methods.
The correlation of PChE and AChE measurements recorded by the kit and those recorded by the WDOH laboratory were quite high. In fact, according to Dr. Keifer, the experiments suggest the kit may measure AChE even more accurately than the laboratory.
Part of the research by Dr. Keifer and his associates evaluated the differences in ChE levels between blood obtained using the finger-stick method, which draws blood from capillaries, and blood drawn from a vein. This was done to determine if skin contamination with pesticides might give falsely low values when finger stick methods were used. On average, the blood from the finger-stick showed slightly lower ChE levels than did blood drawn from the vein. Dr. Keifer says that although the difference between these levels was statistically significant, it was very small relative to the amount of activity change currently considered to be critical, and because of this should not be viewed as clinically significant.
"However," Dr. Keifer adds, "this study data adds to the evidence that skin contamination from pesticides may be a source of error in capillary-based testing, and that attention to hand washing or other skin-cleaning methods is necessary to assure accurate results."
Another finding of the study was that the cholinesterase levels reported by the field-test kit could be affected by temperature. While the kit had an internal program to adjust results for changes in temperature, this program did not appear to adjust the results enough to allow testers to disregard temperature completely. (During the field-testing phase of the study, the testing environment was always within a narrow temperature range, and this avoided undue influence of temperature on the field results.)
Dr. Keifer's study found a limited range of cholinesterase values among the workers tested. Because the values were in the normal range for humans, there appears to have been relatively little heavy exposure to pesticides, Keifer notes.
"While this was excellent news for the workers, the lack of heavy exposure prevented us from determining how the field-test kit performs across a wide range of values. As a result, we can say little about how the kit performs when values are very low in workers tested in the field."
One unexpected finding of Dr. Keifer's study was that quality control for red blood cell cholinesterase testing at the WDOH laboratory was problematic. Results from the laboratory were difficult to interpret and did not match those obtained with the kit. Since the results from the kit were more consistent with the exposure scenario than the results from the laboratory, blinded spiked samples were sent to the laboratory. When kit and lab results from the spiked blood samples were compared side by side, the results from the kit appeared to better reflect the known pesticide content of the spiked samples.
While only a single laboratory was used for comparison to the kit throughout the study, results from a study conducted by the Environmental Protection Agency indicate that methods commonly used by commercial laboratories across the country may give imprecise results.
"Based on the results of our study and those of the EPA study, I am not certain the methods commonly used by many commercial laboratories are capable of providing reliable results," concludes Dr. Keifer.
He goes on to explain that there has been no systematic effort to standardize cholinesterase testing values. "Unlike many common laboratory tests which have quality assurance programs, no systems are in place to assure quality for cholinesterase testing."
It is widely held that workers frequently exposed to OP and carbamate insecticides should be in a cholinesterase testing and monitoring program.
In fact, the state of California has required cholinesterase monitoring since 1974 for all workers with more than a specified amount of exposure to Class I or II OPs or n-methyl carbamates. The objectives of the California program are to be able to remove overexposed workers from exposure situations, to identify and intervene in high-risk work behaviors, to help decide when the exposed worker can safely return to work, to raise awareness of the toxicity of these pesticides among employers and workers, and to prevent chronic health effects from exposure to the cholinesterase-inhibiting materials.
Field-test kits are not currently in widespread use in California. Most growers use commercial laboratories. "(California labs have) probably worked out the bugs more completely than the labs (in Washington)," concedes Dr. Keifer, "because they have been doing it for a lot longer."
Should cholinesterase monitoring be required in Washington? Dr. Keifer points out that such a program imposes a substantial economic burden on growers. But if pesticide poisonings are avoided, a substantial personal and economic burden is avoided for both the grower and the worker. Also, he added that orchardists in Washington do not appear to use insecticides as much or as long as growers in California.
In California, testing is required only for workers who work with Class I and II OPs or carbamates for six or more days in a thirty-day period. By spreading out the application duties among several trained workers, individual exposure days can be reduced, thus avoiding testing under these parameters.
So what's the bottom line for field-test kits? "Under very restricted conditions, (the tested kit) provides potentially valuable information about exposure to cholinesterase-inhibiting substances in an inexpensive and efficient way," says Dr. Keifer. The restricted conditions include the establishment of a standard against which to compare the results, and using the kit within a relatively narrow temperature range. (A new version of the Testmate kit is now available which claims to have solved the temperature variability problem. Dr. Keifer says the PNW Agricultural Safety and Health Center is testing one of the new kits. Other improvements to the newer kits include providing sample buffer bottles that permit a field-drawn sample to be transported to a distant location without drying out.)
"Potentially," Dr. Keifer concludes, "the Testmate kit is a good tool. It has some real potential, partly because it uses proven methodology to conduct the tests."
The Pacific Northwest Agricultural Safety and Health Center, funded by NIOSH, is one of eight such centers in the United States. The Center's mandate is to study occupational health and safety issues in farming, forestry and fishing in the four Region C states of Idaho, Washington, Oregon and Alaska. Dr. Richard Fenske is the Center Director, Dr. Matthew Keifer is Co-Director, and Sharon Morris is Associate Director. Adrienne Hidy is the Center's Administrator. This article was prepared by Norm Herdrich, PNASH Outreach Coordinator. To obtain additional information, he can be contacted at (509) 926-1704 or normh@u.washington.edu.
The PNN is operated by WSU's Pesticide Information Center for the Washington State Commission on Pesticide Registration. The PNN system is designed to distribute pesticide registration and label change information to groups representing Washington's pesticide users. The material below is a summary of the information distributed on the PNN in the past month.
Our office operates a web page called PICOL (Pesticide Information Center On-Line). This provides a label database, status on registrations and other related information. PICOL can be accessed on URL http://picol.cahe.wsu.edu or call our office, (509) 372-7492, for more information.
Note: The purpose of the following PNN notifications was to make members of Washington's agricultural community aware of certain inquires that had been made by USDA. In each case USDA was asking for information based on an initial inquiry from EPA. The information was distributed on the PNN because it may indicate the direction of future regulatory action.
USDA's Office of Pest Management Programs requested that certain states gather information on the possible impact to the strawberry industry if the phi for iprodione was changed from 0 to restrict the use to "first flowering." Iprodione is registered for use on strawberries in Washington under four labels; all are Rhone Poulenc: Rovral, Rovral 4, Rovral 50SP, and Rovral WG. WSU made inquiries of appropriate extension specialists and passed along the following information to USDA:
First, If iprodione is restricted to prebloom application, it will become unusable to the strawberry industry. Growers need to have Rovral as a option at least for the first application at 5 to 10% bloom.
Second, The status of alternatives to Rovral is as follows:
Captan - is effective and has little risk for developing resistance. The PHI is 3 days. Use of this product without the availability of iprodione and its 0-day PHI will alter grower's picking schedule.
Benlate & Topsin M - not effective due to resistance.
Ronilan - manufacturer is in the process of voluntarily deleting strawberries from all product labels.
Thiram - not as effective as captan but useful for managing resistance; however, Whatcom county growers have no experience with thiram.
USDA's Office of Pest Management Programs also requested that certain states gather information on the possible impact to the stone fruit industry if the phi for iprodione was changed from 7 days to either 45 or 90 days. Iprodione is registered for use on stone fruit in Washington under four labels; all are Rhone Poulenc: Rovral, Rovral 4, Rovral 50SP, and Rovral WG. WSU made inquiries of appropriate extension specialists and passed along the following information to USDA:
Changing the phi to 45 or 90 days will preclude using iprodione for the fruit rot phase of brown rot (the phase of most concern); however, effective alternate products do exist. Therefore, changing the iprodione phi to either 45 or 90 days will have little impact on Washington's stone fruit production.
USDA's Office of Pest Management Programs requested information on the possible impact of banning the use of aluminum or magnesium phosphide within 500 feet of any occupied (working or living) structure. Both aluminum phosphide and magnesium phosphide are typically used to fumigate grain or other commodities in storage or transit or to fumigate empty agricultural containers. Other registered uses in Washington include use to fumigate rodent burrows or bee hives. WSU made inquiries of a commercial pest control company that specializes in food processing, export, and warehousing applications. The following information was passed on to USDA:
Imposing a 500 foot buffer zone around phosphine fumigations would severely restrict the use of these products. Because of the pending loss of methyl bromide, after January 1, 2001, phosphine will be the only fumigant registered for use on food commodities. Currently EPA lists aluminum phosphide and magnesium phosphide as alternatives for methyl bromide. Restricting phosphine use via the imposition of a buffer zone will severely limit agriculture's ability to fumigate stored commodities.
In almost no cases will a 500 foot buffer zone exist where fumigation of shipping containers and truck trailers are concerned. In-transit fumigation of stored commodities will not be possible with a buffer zone requirement. Not only will this restriction impact commodities being handled within the US it will also make it difficult to meet the import requirements imposed by other countries.
The final comment passed along to USDA questioned whether off-site exposure is a valid concern.
Section 24c Registrations
First, WSDA issued a revision to SLN WA-940020. This SLN had previously been issued for the use of Novartis' formulation of Supracide 25WP, EPA Registration Number 100-754. The revision includes adding a restricted use pesticide (RUP) statement, changes to the pollinator protection statement, and changing the SLN number to WA-940020a. Novartis plans to transfer the Supracide registration to Gowan in the near future; however, this SLN will remain in effect for at least another year.
Second, because Gowan will be taking over this registration, WSDA issued a second SLN, WA-940020b, to Gowan for their Supracide 25WP formulation, EPA Registration Number 100-754-10163. This SLN is virtually identical to the SLN issued to Novartis.
Section 24c Revisions
Pesticide pre-licensing and recertification courses will be conducted on the following dates. The registration fee for either type of course is $30 early (postmarked 14 days prior to the program), otherwise $45 per day. For information contact: Cooperative Extension Conferences: 509-335-2830 or pest@cahe.wsu.edu. Information is also available on-line at http://pep.wsu.edu. WSU Recertification Courses offer 6 credits per day.
|
Eastern Washington |
Western Washington |
||
| Spokane | January 13 & 14 | Vancouver & PCOWorkshop | January 6 & 7 |
| Yakima | January 21 & 22 | Tacoma | January 13 & 14 |
| Pasco | January 26 & 27 | Edmonds | January 21 & 22 |
| Moses Lake | January 28 & 29 | Port Orchard | January 28 & 29 |
| Pullman | February 3 & 4 | Olympia | February 1 & 2 |
| Wenatchee | February 17 & 18 | Highline | February 4 & 5 |
| Spokane (Agriculture) | February 19 | Mt. Vernon | February 10 & 11 |
| Tacoma | February 24 & 25 | ||
| Seattle | March 4 & 5 | ||
| Bellingham Insect Workshop | March 12 | ||
Washington State University annually conducts pre-license training for pesticide applicators, consultants, and dealers. Washington State Department of Agriculture offers all exam categories at the end of the training. Anyone preparing for pesticide licensing exams will benefit from the training programs offered; however, this training will be most useful to those preparing for the following license exams: Weed Control (Agric., Turf & Ornamental, Rights-of-way); Private Applicator Exam; Insect and Disease Control (Agric., Turf & Ornamental); Dealer Manager Exam; Aquatic (January 26 in Pasco only); and Laws & Safety.
|
Eastern Washington |
Western Washington |
||
| Spokane | January 12, 13, 14 | Vancouver | January 5, 6, 7 |
| Yakima | January 20, 21, 22 | Tacoma | January 12, 13, 14 |
| Pasco | January 25, 26, 27 | Mt. Vernon | February 9, 10, 11 |
| Pullman | February 2, 3, 4 | Tacoma | February 23, 24, 25 |
| Wenatchee | February 16, 17, 18 | Puyallup | March 23, 24, 25 |
| Richland | February 22 | Wenatchee | February 24 |
| Yakima | February 23 | Spokane | February 15 |
| PCO Workshop Vancouver | January 7 | Landscape Insect Workshop, Bellingham | March 12 |
Chemical (type) |
Federal Register |
Tolerance (ppm) |
Commodity (raw) |
Time-Limited |
||
Yes/No |
New/Extension |
Expiration Date |
||||
| alder bark residue | 10/5/98 page 53291 | exempt | see comment | No | N/A | N/A |
| Comment: This exemption applies when alder bark residue is used as an inert ingredient (seed germination stimulator) in pesticide formulations applied to growing crops. | ||||||
| pyridaben | 10/5/98 page 53294 | 0.75 | cranberries | Yes | New | 12/31/99 |
| Comment: This time-limited tolerance is issued in response to EPA granting a Section 18 for the use of pyridaben to control southern red mite in Massachusetts' cranberries. | ||||||
| avermectin (insecticide) | 10/7/98 page 53825 | 0.05 | basil | Yes | Extension | 1/31/00 |
| Comment: This time-limited tolerance is extended in response to EPA granting a Section 18 for the use of avermectin to control leafminers in California basil. | ||||||
| bifenthrin (insecticide) | 10/7/98 page 53818 | 0.5 | canola | Yes | Extension | 3/30/00 |
| Comment: This time-limited tolerance is extended in response to EPA granting Section 18's for the use of bifenthrin to control aphids in canola in Oregon, Washington, and Idaho. | ||||||
|
fludioxonil (fungicide) fludioxonil (fungicide) |
10/7/98 page 53820 10/7/98 page 53820 |
0.01 | brassica, leafy vegetable |
No No |
N/A N/A |
N/A N/A |
| 0.02 | bulb vegetables | |||||
| 0.02 | cereal grains | |||||
| 0.01 | cucurbit vegetables | |||||
| 0.01 | legume vegetable; foliage | |||||
| 0.01 | cereal grains; forage, fodder, and straw | |||||
| 0.01 | fruiting vegetables except cucurbits | |||||
| 0.01 | grass; forage, fodder and hay | |||||
| 0.02 | herbs and spices | |||||
| 0.01 | legume vegetables | |||||
| 0.01 | non-grass animal feeds | |||||
| 0.01 | rape; forage and seed | |||||
| 0.02 | root and tuber vegetables | |||||
| 0.01 | sunflower seed | |||||
| imidacloprid (insecticide) | 10/7/98 page 53826 | 0.3 | beet root | Yes | Extension | 6/30/00 |
| 0.3 | turnip root | |||||
| 3.5 | turnip top | |||||
| Comment: This time-limited tolerance is extended in response to EPA granting a Section 18 for the use of imidacloprid to control green peach aphid in California beet and turnip crops. | ||||||
| pyridate (fungicide) | 10/7/98 page 53837 | 0.1 | chickpea | No | N/A | N/A |
| tebuconazole (fungicide) | 10/7/98 page 53813 | 0.2 | sunflower seed | Yes | Extension | 9/30/99 |
| 0.4 | sunflower oil | |||||
| Comment: This time-limited tolerance is extended in response to EPA granting Section 18's for the use of tebuconazole to control rust in sunflower crops in North Dakota, Kansas, Colorado, and Nebraska. | ||||||
|
sethoxydim (herbicide) sethoxydim (herbicide) |
10/8/98 page 54066 10/8/98 page 54066 |
0.2 | apricot |
No No |
N/A N/A |
N/A N/A |
| 0.2 | cherry | |||||
| 0.2 | nectarine | |||||
| 0.2 | peach | |||||
| 15 | bean; forage and succulent | |||||
| 16 | soybean | |||||
| 1 | grape | |||||
| 2 | raisin | |||||
| 4 | cilantro | |||||
| 4 | leafy vegetable crop group (except Brassica) | |||||
| 4 | tuberous and corm vegetable subgroup | |||||
| 1 | garden beets | |||||
| 5 | cranberry crop subgroup | |||||
| 5 | globe artichoke | |||||
| cyromazine (insecticide) | 10/9/98 page 54360 | 0.05 | turkey; meat, fat, and mbp | Yes | Extension | 4/1/00 |
| Comment: This time-limited tolerance is extended in response to EPA granting Section 18's for the use of cyromazine to control flies on turkeys in North and South Carolina. | ||||||
| mancozeb (fungicide) | 10/9/98 page 54362 | 2 | ginseng | Yes | New | 12/31/99 |
| Comment: This time-limited tolerance is established in response to EPA's granting a Section 18 for the use of mancozeb to control leaf and stem blight in ginseng grown in Wisconsin. | ||||||
| paraquat (herbicide/desiccant/defoliant) | 10/9/98 page 54357 | 0.3 | dry peas | Yes | Extension | 5/15/00 |
| Comment: This time-limited tolerance is extended in response to EPA granting Section 18's for the use of paraquat to control weeds in dry peas grown in North Dakota, Montana, Idaho, Washington, and Oregon. | ||||||
| dimethomorph (fungicide) | 10/13/98 page 54587 | 0.05 | potatoes | No | N/A | N/A |
| hexythiazox (insecticide) | 10/13/98 page 54594 | 2 | hop | Yes | New | 9/15/00 |
| 3 | strawberry | |||||
| Comment: This time-limited tolerance is established in response to EPA granting Section 18's for the use of hexythiazox on California strawberries and on hops grown in Washington, Oregon, and Idaho. | ||||||
| azoxystrobin (fungicide) | 10/16/98 page 55533 | 0.03 | potatoes | Yes | New | 10/18/99 |
| Comment: This tolerance was requested by Wisconsin potato growers, University extension specialists, Zeneca, and EPA in an effort to gather data to support registration of a reduced-risk fungicide. | ||||||
| hexythiazox (insecticide) | 10/16/98 page 55540 | 2 | dried hops | No | N/A | N/A |
Miscellaneous Information |
||||||
| On October 9, 1998, EPA announced that, in response to FQPA requirements, the agency had established a policy in conjunction with the FDA that 1) established interpretations of the FFDCA as they relate to jurisdiction of EPA and FDA over antimicrobial substances used in food; 2) discussed interpretation of certain terms in FIFRA relevant to the authority of the two agencies; 3) described how EPA and FDA propose to clarify the post-FQPA regulatory authority over certain antimicrobial substances; and 4) discussed how EPA and FDA plan to handle the review of petitions for antimicrobial substances that will remain under EPA jurisdiction and for those that EPA proposes to return to FDA's authority. (10/9/98 page 54532) | ||||||
| On October 20,1998, the Agricultural Marketing Service announced that it is proposing to revise the Federal Seed Act (FSA). The proposed changes include prohibiting shipment of agricultural and vegetable seeds containing seeds of noxious weeds, adding two kinds to the list of those subject to the FSA, and updating the seed testing and certification regulations. (10/20/98 page 55964) | ||||||
| In the October 29 Federal Register, EPA announced a schedule for issuance of a series of scientific policies that will be used to comply with the provisions of the Food Quality Protection Act. (10/29/98 page 58038) | ||||||