
December 2002, Issue No. 200
A monthly report on environmental
and pesticide related issues
In This Issue
Parasites,
Pesticides, and Polliwogs: Hazards versus Risk
|
|
| |
|
| |
|
|
Parasites,
Pesticides, and Polliwogs: Hazards versus Risk |
|
Open Forum: In an attempt to promote free and open discussion of issues, Agrichemical and Environmental News encourages letters and articles with differing views. To discuss submission of an article, please contact Dr. Allan Felsot at (509) 372-7365 or afelsot@tricity.wsu.edu; Dr. Catherine Daniels at (509) 372-7495 or cdaniels@tricity.wsu.edu; Dr. Doug Walsh at (509) 786-2226 or dwalsh@tricity.wsu.edu; Dr. Vincent Hebert at (509) 372-7393 or vhebert@tricity.wsu.edu; or AENews editor Sally O'Neal Coates at (509) 372-7378 or scoates@tricity.wsu.edu. EDITORIAL POLICY, GUIDELINES FOR SUBMISSION.
This
issue has been viewed
times since December 6, 2002.
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Problematic Product Names
A QBL Mini-Rant
Jane M. Thomas, Pesticide Notification Network Coordinator, WSU
(ED. NOTE: In an ongoing effort to elicit a job offer from the U.S. Environmental Protection Agency (EPA), Her Royal Highness The Queen Bee of Labels (HRH QBL, a.k.a. WSU's Jane M. Thomas) takes time out of her busy Royal Schedule periodically to point out oddities and aggravations on pesticide labels. It is the QBL's Opinion Most High that if she were in charge of all things label, a few RULES, combined with swift and thorough consequences for transgressors, would whip the whole pesticide label business into shape in a matter of weeks. Until such time as EPA sees the light and appoints HRH QBL to her rightful position, The Queen shall content herself with commentaries such as the following article.)
Last spring EPA announced that it was issuing a draft Pesticide Registration (“PR”) notice for public comment covering the topic of false or misleading pesticide product brand names. Although not normally prone to moments of religious zeal, I did figuratively descend from my throne long enough to utter a (dignified) Hallelujah! As my Loyal Followers will remember, way back in May of 2000 (Issue No. 169 of this fine newsletter) in my inaugural article “If I Were the Queen of Labels,” I proposed the original nearly 12-step Get Well Program for pesticide labels. Dedicated Readers might recall that this list of label Dos and Don'ts included Royal Rule Number 7 concerning Product Names. To wit: “Registrants shall clearly identify the product name; names shall not be misleading or confusing.”
Meeting of the Minds?
I was delighted when I first saw the aforementioned EPA announcement, thinking “It has finally happened: The QBL and EPA have had a mind meld! Both are working on a subject near and dear to the Royal Heart.” Alas, upon closer inspection, I quickly came to the conclusion that while we were both on the same subject we were on different wavelengths, or to use a bad analogy, although we were in the same castle, we were in different turrets.
The EPA document (available at URL: http://www.epa.gov/opppmsd1/PR_Notices/#eup_review) provides guidance on the subject of misleading product names. It offers examples of statements associated with product names considered to be false or misleading by EPA. The statements fall into three basic categories.
- Composition (e.g., "100% Pure," "Contains No XXX")
- Effectiveness (e.g., "100% Protection," "Germ-Shield”)
- Safety (e.g., "Safe," "Non-Toxic," "Environmentally Safe")
Well, so much for my bonding with EPA. While these are all valid concerns with respect to pesticide product names, I think that EPA needs to get back to basics.
Back to Basics
What about the product name itself? Shouldn’t the name be obvious when looking at the label? Shouldn’t the name on the label be the same as the product's registered name? We here at the Washington State Pest Management Resource Service (WSPRS) think these basic issues deserve attention. We like to think we are at least as smart as the average label reader, but the fact is that, in some cases, we are a bit stymied when trying to sort out exactly what the product name IS. Indeed, matching a label with a name on a product registration sheet can be surprisingly daunting.
Mysterious Mildew Monikers
Late last year, while rummaging around in the files, I came across the following two Clorox labels, both carrying EPA Registration number 5813-24. According to Clorox, one product is called Tilex Instant Mildew Remover and the other is Tilex Disinfects Instant Mildew Remover. Take a look at the container labels and you tell me if you can identify which label is associated with which name.
 |
 |
Next, imagine the confusion in our office when 2002 rolled around and we received the 2002 Clorox pesticide registration sheets. Clorox did not register Tilex Instant Mildew Remover in 2002. Now the keen staff must correctly identify the Tilex Instant Mildew Remover label so that it can be tossed. I should set your minds at ease: we got it right (of course), but Clorox did not make this easy. Far be it from Moi to criticize Clorox. Naturally, I do not sully the Royal Hand by personally using any of their fine products, but the staff at the castle is fond of Clorox and would be very unhappy to think that I was casting aspersions on the Clorox Character.

Just to add further confusion to the Clorox Conundrum there is one more tiny thing. The following label, also EPA registration number 5813-24, is also currently registered. Note that, according to Clorox, the name for this product is Lemon Fresh Tilex Disinfects Mildew Remover. Note further that nowhere on the label does the word "Disinfects" appear. Is it too much to ask that a product’s name be clearly identified on the product label and that it agrees with the name being used by the registrant on its registration sheet? While I shudder to make such a suggestion, if Clorox wants to produce a host of different labels for the same product, why not simply call these labels Formula I, Formula II, and so forth, so that they are clearly identifiable?
Redundant Repellant
An equally confusing but opposite example comes from SC Johnson. Here are two labels that cohabitated in the files last year.
 |
 |
Naturally, you and I would expect that the products would be named “Off! Deep Woods” and “Deep Woods Off!” Not so, according to SC Johnson. They think that both products are “Deep Woods Off!” And since these are in fact SC Johnson's products, we have no choice but to accept their word for what are, in fact, the correct product names. (Thank heavens SC Johnson isn't a parent. With this penchant for redundant name selection, they also might end up with a host of children all named George Foreman.) But back to the topic at hand. I ask again: is it really too much to expect that a product name have some connection to the wording that appears on the actual label?
Adjuvant Antics
This next example from Wilbur-Ellis is perhaps a bit more in keeping with EPA's concerns. The product in question is an adjuvant named PH. (HRH gratefully acknowledges WSDA's ever-vigilant Erik Johansen for bringing this label to the Royal Attention.)
As you can see from the label information shown here, this product does not function in any way, shape, or form as a buffering agent as one might expect from the name. Is this name misleading? Yes. But rather than being misleading with respect to EPA’s concerns of composition, effectiveness or safety, it is misleading with respect to function.

I hear some cries of "foul" because this product is not currently registered (thank heavens for small things) and "do-overs" because this is just an adjuvant and everyone knows that adjuvants are mere distant cousins to pesticides. Everyone please remain calm. A lousy label example is a lousy label example regardless of whether it is an adjuvant and regardless of registration status. As such, it deserves to be called on the Royal Carpet in this newsletter. It’s nothing personal, Wilbur-Ellis. I am the Queen. It’s what I do.
A Rose Dust by Any Other Name
My final example, for now, of pesticide product name problems is from Green Light, who had the following two labels registered in the state of Washington last year. As you can see, the EPA registration numbers are different. The 572-185-869 product has been registered for some time while the 4-30-869 label was added as a new registration late in 2001. (While Green Light had not indicated that 572-185-869 was in discontinuance in 2001 it was not reregistered in 2002.)
 |
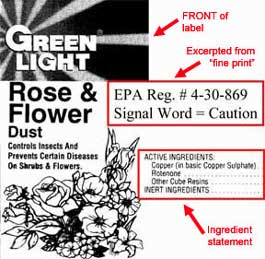 |
Please take note of the similarity of the two labels. Now imagine yourself to be the poor homeowner who has run out of your supply of trusty Rose & Flower Dust. You go to the store and purchase a new container of dust and you assume that you have replaced your old product. NOT SO. The older version of the product, EPA registration number 572-185-869, contains malathion, methoxychlor, and captan and carries the signal word “danger.” The newer product contains copper and rotenone and carries the signal word “caution.” These two products, although packaged identically, right down to the graphic on the label, are night and day.
Now I will admit that this example likely stems from a company phasing in a new formulation and thus a new label while phasing out another. If this is the case, why didn't Green Light simply include something like "New Formulation" on their label to alert buyers that they are purchasing a product different from the one with which they are familiar?
Royal wRap-Up: Registrant Responsibility
I am FINALLY finished on the subject of product names. For now. I do realize that I have once again gone on and on (and on) in a modified Royal Rant. But please understand, Loyal Reader, that I was so excited to think, for an instant, that EPA and I shared the dream. I envisioned us yoked, shoulder-to-shoulder, pulling together in the traces, working together for a common cause. But no. While I agree that there are many and several problems with pesticide product names, none of the problems I cite with pesticide product names have to do with statements about composition, effectiveness, or safety. These are valid concerns, but I assert that EPA's guidance document has not gone far enough.
So let’s put EPA aside for a moment. Registrants, it is up to you! Shape up. Stop using misleading product names. Start clearly identifying the product name on the label. Give the WSPRS staff and the consumers a break! You know I didn't just write the Royal Rules so that I could hear the keyboard click...
Jane M. Thomas, a.k.a. HRH QBL, reigns from the office of Washington State Pest Management Resource Service (WSPRS), formerly known as the Pesticide Information Center (PIC), on the Tri-Cities Campus of Washington State University. She can be reached at (509) 372-7493 or jmthomas@tricity.wsu.edu .
Go to this issue's Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
FEQL Advisory Board Meets
Brochure to Be Developed, Water Monitoring Discussed
Dave Winckler, Chair, FEQL Advisory Board
The Food and Environmental Quality Laboratory (FEQL) at Washington State University (WSU) held its fall Advisory Board meeting on Wednesday, October 23, 2002, on the Tri-Cities campus of WSU. Highlights of this meeting included the decision to develop a brochure or informational statement describing FEQL and a discussion of Washington State Department of Agriculture’s surface water monitoring program.
Background
The FEQL was mandated by the Washington State Legislature to focus research and extension efforts on all aspects of crop protection technologies across the state. The need for such a WSU facility grew out of two concerns: potentially devastating losses of crop protection tools for the minor crops characteristic of Washington agriculture and the safety of these tools to human health and the environment. In accordance with the founding legislation, FEQL is advised by a board of stakeholders representing a number of distinct functions pertaining to Washington State agriculture. For example, food processing, health care, farm labor, marketing, and environmental protection are among the constituencies represented on the board.
I serve as the third elected chair of this advisory board, having assumed this role last June from Marilyn Perkins. I work with the Washington Farm Bureau and fill the position of farm organization representative on the board; Marilyn is with the League of Women Voters and represents the consumer constituency. The board formally recognized Marilyn’s term of office by presenting her with a plaque at this October meeting.
Budget Update
Ralph Cavalieri, Associate Dean of the College of Agriculture and Home Economics of Washington State University, formally represents the university on the board. Ralph presented a discussion of the WSU budget, including the sizeable impacts on various agriculture-related programs and projects resulting from recent state budget cuts. While the FEQL was not affected directly, the entire university system feels the effects of the increased workload on remaining staff members. In an effort to inform legislators and the public about the importance of non-classroom functions of research institutions (i.e., research and extension), the presidents of WSU and University of Washington are joining forces in an unprecedented partnership to increase awareness of such programs and their impact. In the aftermath of absorbing the budget cuts, WSU has proposed a four-pronged funding approach to Governor Locke:
- a $36 million funding increase over the biennium (core funding, to be used for salaries, high-priority initiatives such as biotech and viticulture);
- $15 million to allow for an increasing enrollment (WSU has accepted more qualified students than the state currently funds);
- $2 million to assist in adjusting to the new relationship between Oregon State University and WSU’s veterinary schools (OSU will no longer send and pay for two years of veterinary school for their students at WSU);
- $600,000 to fill staff positions for increased state and federal administrative demands. The board discussed state budget priorities in general and how this message translated to support of FEQL. Many board members favored the idea of FEQL packaging and presenting information about its many successes to members of the state legislature.
WDOE Report
Don Abbott, who holds the board seat reserved for the Washington State Department of Ecology, gave a presentation about his agency’s activities. WDOE’s lead arsenate task force has been meeting in an effort to come up with solutions for landowners with contaminated land. (ED. NOTE: Lead arsenate was widely used as a pesticide in orchards prior to the 1960s. It is extremely persistent in soil. Many former orchard sites have been converted to housing, schools, and other non-agricultural uses.) Sampling has been ongoing in the Wenatchee area, Washington’s main tree fruit-growing region, revealing schoolyards with higher than natural levels of lead and arsenic in their soil; mitigation efforts are being investigated and are targeted for the coming year. The Department of Health has been cooperating in toxicology studies on children at these schools. Other sources of lead and arsenic in children are being investigated as well.
FEQL Faculty Reports
Allan Felsot, FEQL’s Environmental Toxicologist, presented a short slide show on “Reduction of Pesticide Inputs, Worker Exposure, and Drift through Alternative Sprayer Technology,” detailing one of the laboratory’s projects this past summer. Funded by the Washington State Tree Fruit Research Commission, the project compared traditional air-blast sprayers with a newer sprayer technology known as “Proptec.”
Vince Hebert, FEQL’s Analytical Chemist, presented an overview of FEQL’s regulatory science program. Compliance with Good Laboratory Practices (GLP) is a complex process and has reached maturity in the FEQL, with roles and specialization spread out among several staff members. He outlined some of the studies that have been undertaken in the lab this year and those planned for the coming year.
Catherine Daniels, WSU’s Pesticide Coordinator and FEQL faculty member, discussed recent developments in her office, including its name change from the Pesticide Information Center (PIC) to Washington State Pest Management Resource Service (WSPRS) (see related article in last month’s AENews) and the launch of its new Website at http://wsprs.wsu.edu . WSPRS has also created and received funding for a brand-new Comment Coordinator position. The Comment Coordinator will function as an information conduit between agricultural producers in the Pacific Northwest and Federal regulators by soliciting comments from grower groups and researchers in response to proposed EPA actions, then presenting a coordinated, complete, and unified set of comments to Federal regulatory agencies. Five-year WSU employee Jane M. Thomas (who also functions as Pesticide Notification Network Coordinator, see http://www.pnn.wsu.edu) assumed the role of Comment Coordinator September 16, 2002.
Water Quality Presentation
In keeping with the board’s decision to use the afternoon of their meetings to focus on a particular issue relevant to Washington agriculture, guest speaker Jim Cowles from Washington State Department of Agriculture (WSDA) made a presentation on the theme of water quality. Jim is an Environmental Toxicologist with WSDA’s Endangered Species Program. Jim’s slide show, “WSDA Surface Water Monitoring,” explained how WSDA proactively established their Endangered Species Program in recognition that the lands defined as endangered species habitat in Washington are largely the same lands on which agricultural production occurs. It detailed the process through which WSDA will gather information and make decisions regarding pesticide use. Jim encouraged collaboration in specific areas of research, analysis, and information dissemination between WSDA and WSU/FEQL as this program develops.
Communicating to the Legislators
It had been suggested at several Advisory Board meetings that the FEQL make a report to the state legislature about its accomplishments. Such a report could take the form of a brochure designed to raise awareness of what FEQL does and how its programs can be a resource for Washington agriculture. After discussing the pros and cons of several approaches, an ad hoc committee consisting of Don Abbott, Scott McKinnie, the FEQL faculty, and me was organized to meet and prepare a draft statement for informal presentation to legislators via legislative aides. The resulting statement will emphasize FEQL as a resource to legislators and policy makers, will support the fact that WSU does many positive things for grower groups, and will have the additional utility of being on hand should such statements be requested by other agencies.
Advisory Board Goes High-Tech?
The spring meeting of the FEQL Advisory Board will be Wednesday, April 23, 2003, at WSU’s Richland campus. As the fall meeting has historically presented logistic problems due to inclement weather, it was suggested that, in 2003, it be held in two locations (western Washington and eastern Washington), linked by satellite. Can the board of one of the most technologically advanced analytical laboratories in the Pacific Northwest achieve that level of communication sophistication? We shall see.
Dave Winckler is the Marketing Specialist at the Washington Farm Bureau and current chair of the FEQL Advisory Board. He can be reached at dwinckler@wsfb.com or (509) 899-1795. For information about the FEQL, contact Doria Monter-Rogers at monter@tricity.wsu.edu or (509) 372-7462.
Go to this issue's Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Pesticides, Parasites, and Pollywogs
Hazards Versus Risks
Dr. Allan S. Felsot, Environmental Toxicologist, WSU
Public perception will forever link pesticides with Rachel Carson’s metaphor of a silent spring. Images of landscapes absent of birds will overshadow any benefits that pesticides have shown in stabilizing food production by protecting yields against a myriad of pests. After the “bird killer” DDT was vanquished, raptorial and fish-eating bird populations rebounded surprisingly rapidly. Since then society has enjoyed the cacophony of birds only occasionally knocked cold by the neurotoxic insecticides still on the market.
Throughout the early history of modern pest control by synthetics, herbicides were untouched by the infamy assigned to insecticides. Today, however, Carson’s vernal silence has extended beyond the chirping of birds to include the croaking of frogs, and worldwide amphibian population declines have been associated with herbicides commonly used in field crop production. It seems that atrazine, especially, has been branded as the new DDT, with recent papers (Hayes et al. 2002a,b; Kiesecker 2002) claiming it is hazardous to pollywogs.
A Tale of Two Maladies
Actually, there are two stories in the amphibian controversy, one involving death or population decline and one involving mutation or other sublethal effects. But the two issues have become intermingled and thus confused.
First, the population issue. Amphibian declines have been documented around the world (Alford and Richards 1999, Young et al. 2001). The laundry list of proposed factors precipitating the population crashes includes
- increases in ultraviolet (UV) radiation due to ozone holes;
- increased predation due to the introduction of exotic predatory fish;
- competition with introduced amphibian species;
- habitat modification including removal of trees, drainage of wetlands, and changes in vegetation structure;
- changes in water quality (e.g., changes in pH, contamination by synthetic chemicals including pesticides);
- increased incidence of parasitism and disease;
- global climate change;
- synergistic interactions among any of these factors.
Ironically, the major amphibian population crashes have occurred in comparatively pristine habitats at higher elevations (Carey 2000). Thus, human-associated factors such as pesticides or habitat modification are not good hypotheses. However, evidence has accumulated supporting the prevalence of a virulent pathogenic fungus (Batrachochytrium dendrobatidis) in diverse places like Australia (Berger et al. 1998) and the mountains of Central America (Carey 2000). The fungus attacks the toughened skin of juveniles and adults after metamorphosis, and the frogs seem to lack immunocompetence to fight the fast-developing infection (Carey 2000).
The second issue involves mutation, malformation, and other sublethal effects. This issue is supported and exacerbated by compelling pictures of school kids holding multi-legged and eyeless frogs collected from agriculturally dominated habitats. Hypotheses of human-induced toxicosis (e.g., Ouellet et al. 1997; Burkhardt et al. 1998) have appeared faster than the flick of a frog’s tongue catching a fly. Concerns rose to a feverous pitch in Minnesota, the state where developmentally challenged frogs first made headlines, probably because University of Minnesota researchers had “linked” the incidence of birth defects with the use of pesticides (suggested by Kavlock 1998 in reference to Garry et al. 1996).
Recent research has shown experimentally and in the field that a trematode (parasitic flatworm, Ribeiroia spp.) infects tadpoles and may be the most widespread cause of limb deformities in collected frogs (Johnson et al. 1999, 2001, 2002; Kiesecker 2002). The parasite’s primary hosts are snails (especially Planorbella spp.), but the trematode larvae migrate from the snail and burrow into the tadpoles in tissues destined for limb generation. The parasite hypothesis was further expanded to suggest that pesticides like atrazine, malathion, and esfenvalerate may increase frog susceptibility to infection by the trematode (Kiesecker 2002). However, the laboratory experiments generating the hypothesis tested pesticide concentrations over exposure periods far above anything plausible from field runoff into ponds. An ecoepidemiological investigation of trematode-infected frogs in the Pacific Northwest and the prevalence of limb malformations suggested that nutrient enrichment of ponds encouraged proliferation of snail populations and thus augmented trematode populations (Johnson et al. 2002). The most susceptible habitat seemed to be artificial impoundments associated with pastures used by dairy cattle.
According to the Web site of the North American Reporting Center for Amphibian Malformations, deformed frogs of all kinds have now been reported in most states. The Midwestern Corn Belt seems particularly inflicted with reports of less than ambulatory amphibians, but the corridor extending from the Pacific Northwest south into California also report a lot of sightings (Figure 1). In Washington State, 12 counties have reported at least one site with collections of malformed frogs. As publicity waxed from the reports in Minnesota, interest in looking for malformed frogs also took off. Examination of the report dates in Washington, for example, shows most occurred during 1999. However, Cascades frogs (Rana cascadae) were sighted with abnormal hind limbs in King County during 1971.
FIGURE 1 |
 |
Location of sites in the Pacific Northwest where malformed frogs have been reported. Source: http://www.npwrc.usgs.gov/narcam/reports/REPORTS.HTM |
Although every author likes to take credit for placing one more piece into the puzzle of disappearing amphibians, the phenomenon of worldwide amphibian population crashes cannot be explained by the prevalence of malformed frogs (Carey 2000). Where individual frogs have been collected and documented in a scientifically designed field survey (e.g., Canfield et al. 2000; Johnson et al. 2002), the incidence of malformations is too low to account for disappearing populations. Studies in Minnesota, for example, show that malformation rates in Northern leopard frogs (Rana pipiens) can be as high as 20% and in the mink frog as high as 75%, but these are the extremes from one pond and are only seen in captured juveniles, not breeding adults. Furthermore the incidence rate varies from site to site and time of year, and the type of malformations (limbs, digits, eyes) are also variable. Average malformation rates are generally under 10%. But developmentally challenged frogs have always been present, just not at the seemingly high numbers observed mostly during the fall months (September and October) in Minnesota (Ouellet et al. 1997, Johnson et al. 2002).
Malformed frogs may be reproductively challenged, therefore less likely to successfully mate, let alone survive predators, parasites, and the rapidly changing conditions of the pond or wetland habitat. The significance of such individuals to the survival of populations in the isolated, man-made ponds of the Corn Belt or the extensive wetlands of the coastal Pacific Northwest is obscure. Pertinently, only a small percentage (i.e., <10%) of eggs laid by one female actually survive the rigors of development to become a sexually mature adult (Carey and Bryant 1995). Thus, in terms of maintaining a population, conserving breeding adults, which can lay hundreds to thousands of eggs, is more important than worrying about larvae (tadpoles) or a comparatively few malformed individuals. In the populations that have precipitously crashed, malformed frogs were decidedly not a causative factor (Carey 2000) nor were habitat modifications obvious.
The New Hypothesis on the Block
The latest hypothesis to explain declining amphibian populations suggests that herbicides at environmentally relevant concentrations are interfering with normal sexual development (Hayes et al. 2002a,b,c). Atrazine in particular has been blamed for a condition in frogs called intersex (aka hermaphroditism) that is characterized by the presence of both male and female gonads in a single individual. The frogs in question structurally appeared male. The reproductive success of such individuals is not known, but intersex individuals have been documented in the field (Reeder et al. 1998).
Atrazine is a common herbicide ingredient. Residues have been detected in water collected from urban and agricultural watersheds all over the United States, so potential exposure of frogs is likely (Larson et al. 1999). Here in Washington State, herbicides containing atrazine are labeled for use on a variety of crops and sites including corn, conifers, turf, and rights of way.
Laboratory studies suggest that atrazine can disrupt normal sexual development, perhaps through direct effects on the endocrine system. So while this new hazard has been identified for atrazine, we do not yet know the risk (i.e., likelihood or probability) that environmental concentrations of atrazine could actually affect frog populations in the wild.
Some in our society would argue for implementation of the precautionary principle, immediately suspending use of atrazine until any possibility of adverse effects could be conclusively ruled out. I propose that a more logical approach would be to figure out whether the risk of adverse atrazine effects is even worth worrying about. To do this, we would take a risk assessment approach.
Risk Assessment Paradigm
Risk assessment as applied to pesticide technology separates the element of hazard from the element of risk and examines both. “Hazard” is potential harm inherent in the toxicity of a substance, but hazard cannot be manifested as harm without exposure. “Risk” is the probability that harm will occur; it depends upon the magnitude of exposure. “Risk assessment” includes the determination of the potential hazards of a substance and the probability that it will cause harm to the environment and/or human health when it is used in the manner for which it is intended.
Risk assessment consists of four parts: hazard identification, dose-response characterization, exposure assessment, and risk characterization. The first step in risk assessment is defining the problem to be analyzed. In this discussion, we want to know the likelihood that atrazine residues in the environment can cause the types of reproductive problems observed in the laboratory.
In Search of the Most Sensitive Endpoint
The purpose of hazard identification is to determine the array of possible physiological and biochemical effects on an organism that could potentially adversely affect its growth, development, and reproduction. Once the array of possible adverse responses is characterized, the risk assessor searches for the highest dose failing to cause the effect.
For mammalian risk assessment, we examine systemic effects such as tissue pathologies, weight loss, or enzyme dysfunction. The toxicological endpoint for the most sensitive malady is known as the NOAEL (No Observable Adverse Effect Level).
For ecological risk assessment, we frequently seek both acute (short-term high-dose) and chronic (longer-term, more modest dose) data. Acute exposure can be expressed in a measurement called the LC50, which is the concentration that proves lethal to 50% of the tested animals. For longer-term exposures (chronic toxicity) we might examine a less drastic endpoint such as reproductive life cycles. This measurement can be expressed as the NOEC (No Observable Adverse Effects Concentration), the highest concentration causing no impairment.
Based on studies of acute toxicity, milligram-per-liter (mg/L) levels of atrazine are necessary to cause death in tadpoles. (This would be an extremely high concentration; normal application conditions outside the laboratory could not result in such a concentration.) The LC50 based on mortality depends on the species tested. One of the most susceptible species tested is the American toad (Bufo americanus); the LC50 of atrazine to late stage tadpoles was estimated to be 10.7 mg/L (Howe et al. 1998). For an even more sensitive endpoint, tadpole deformities, the NOEC was 2.6 mg/L (Allran and Karasov 2001).
Two independent studies have shown that African clawed frog (Xenopus laevis) tadpoles exposed to atrazine in water suffer from either intersex condition or impaired testicular development when they metamorphose into the juvenile form (Hayes et al. 2002a; Tavera-Mendoza 2002). These are plausible hazards because atrazine can induce (enhance the activity of) aromatase, the enzyme that converts testosterone to estrogen (Crain et al. 1997; Sanderson et al. 2001; Sanderson et al. 2002). If genetically male animals do not have enough circulating testosterone, their gonads will develop abnormalities in sperm production and/or female structural characteristics. Female frogs seem unimpaired by atrazine. Researchers thus far have not tested whether intersex males can mate normally and successfully reproduce, so ecologically relevant hazards of atrazine have not been tested. However, hermaphroditic frogs have been collected from seven locations across the United States, and all locations had detectable, but variable, concentrations of atrazine (Hayes et al. 2002b,c).
Deceptive Dose Data
In contrast to the mg/L concentrations causing frog mortality and malformations, much smaller microgram-per-liter (µg/L) concentrations seem to adversely affect the endocrine system and lead to sexual impairment. In African clawed frogs exposed to atrazine from egg hatch until metamorphosis (about 1.5 months), intersexuality was observed in 16-20% of exposed males over a concentration range of 0.1 µg/L through 25 µg/L (Hayes et al. 2002). These results are unusual because no defined dose-response function was obtained. In other words, the severity of toxicological effects usually increases with increasing dose, but in the Hayes et al. study (2002), the response was the same at all doses. Nevertheless, no effect on sexual development was observed at the lowest dose tested, 0.01 µg/L. Another study showed effects on testes development at 21 µg/L, but this was the only dose tested (Tavera-Mendoza).
The picture of sexually confused frogs was recently clouded further by surprising results from laboratory studies of the American leopard frog, Rana pipiens (Hayes 2002c). When exposed to either 0.1 µg/L or 25 µg/L atrazine in water right after hatch through metamorphosis, juvenile male leopard frogs exhibited testicular anomalies including gonadal dysgenesis (abnormal testis and sperm development) and testicular oogenesis (presence of eggs in the testes). Paradoxically, the greatest percentage of testicular malformations was observed in the lower (0.1 µg/L) treatment rather than the higher (25 µg/L) treatment.
Although these data on leopard frogs tempt the hypothesis that the typical dose-response relationship is turned on its head, critics have already pointed out problems with the report (Carr et al. 2002). In addition to lacking any statistical analysis and presentation of variability among measurements, the data are not consistent with the observations from the African clawed frog: an essentially constant frequency of testicular abnormalities (Hayes et al. 2002a). Also, two doses are not adequate to make any conclusions about the shape of the dose-response relationship. Nevertheless, under laboratory conditions, atrazine seems the plausible cause of developmental problems in male gonads.
In examining the dose-response relationship, it is also worth noting that Syngenta, the registrant of atrazine, conducted its own laboratory experiments to determine the relationship between dose and intersex. The company reported to EPA that it found no effect in the African clawed frog (X. laevis) after exposure to concentrations ranging from 0.01 to 25 µg/L (reported in U.S. EPA 2002a). EPA commented that Syngenta’s data were preliminary and lacked sufficient detail for comparison to the published studies of Hayes et al. 2002.
Under the risk assessment paradigm the most sensitive toxicological endpoint would be the development of the intersex condition, and the NOEC associated with this endpoint is the empirically observed concentration of 0.01 µg/L atrazine based on the study with the African clawed frog (Hayes et al. 2002a). The actual NOEC is somewhere between 0.01 and 0.1 µg/L but was not empirically determined. Often, in the absence of a valid NOEC, EPA will use the lowest observable effect concentration (LOEC) and divide it by a factor of 3. Thus, it would not be unreasonable to assume an effective NOEC of 0.03 µg/L for the toxicological endpoint of intersex in laboratory-exposed frogs.
Atrazine Exposure Characterization
Having done our best with hazard identification and dose-response characterization, the next step is to determine the level of exposure, and thus the likelihood (or how often) such exposures will be realized. Two sources of exposure information can be used. EPA determines environmental exposure by running computer models to estimate atrazine concentrations (called EECs, expected environmental concentrations) in a stagnant pond of water 2 meters (6 feet) deep and 1 hectare (0.47 acres) in surface area. The other source of useable data is the USGS National Water Quality Assessment Project (NAWQA) (Larson et al. 1999). The NAWQA Project has monitored pesticide residues in 36 major agricultural and urban watershed basins of the United States. The NAWQA focus has been streams and rivers rather than ponds, so its relevance to frog populations breeding in ponds and wetlands may be somewhat questionable. However, the NAWQA data do represent runoff-derived residues. On-farm ponds would periodically experience direct pesticide runoff and wetlands would be drainage sinks for streams that also received runoff.
The latest draft of EPA’s Registration Eligibility Decision Document (RED) for atrazine has presented both the modeled atrazine residues and in a semi-probabilistic form, the distribution of atrazine residues from the USGS NAWQA program. The residue concentrations shown in Table 1 represent the EECs determined by computer modeling of three application scenarios to different crops. Note that EPA has assumed very little dissipation of atrazine from the virtual ponds because the concentration 90 days after application differed little from peak (or initial) concentrations.
TABLE 1 |
||||||
| Crop | Application
Rate (lb/A) |
Peak
Concentration |
4-day
Average |
21-day
Average |
60-day
Average |
90-day
Average |
| Corn | 2 |
38.2 |
38 |
37.2 |
35.5 |
34.2 |
1.1 |
21 |
20.9 |
20.5 |
17.7 |
18.8 |
|
| Sorghum | 2 |
72.7 |
72.3 |
70.6 |
67.7 |
65.9 |
1.2 |
43.6 |
43.4 |
42.4 |
40.6 |
39.5 |
|
| Sugarcane | 4 |
205 |
204 |
202 |
198 |
194 |
2.6 |
133 |
133 |
131 |
129 |
126 |
|
Estimated environmental concentrations (EECs, µg/L) of atrazine based on EPA computer modeling using a pond as the target aquatic system (data from U.S. EPA 2002b). |
||||||
The residues shown in Table 2 represent data generated by the USGS from water samples collected across the United States. The data are expressed as percentiles of concentration. For example, the median concentration (50th percentile) for atrazine from the entire agricultural watershed database is 0.027 µg/L. Thus, half of the collected water samples can be expected to be above this concentration, and half below it. At the 95th percentile, only 5% of the concentrations would exceed 3.25 µg/L.
TABLE 2 |
|||||
| Basin Type | Maximum
µg/L |
99th
Percentile |
95th
Percentile |
90th
Percentile |
50th
Percentile |
| Agriculture | 120 |
13 |
3.25 |
1.2 |
0.027 |
| Urban | 14 |
2.75 |
0.65 |
0.33 |
0.041 |
| Integrator* | 27 |
12.5 |
5.35 |
1.95 |
0.062 |
| *This classification includes the rivers and reservoirs into which smaller bodies of water flow. It represents the integration of waters from both urban and agricultural watersheds. | |||||
| Distribution of measured atrazine residues reported as percentile concentrations (µg/L) in the USGS NAWQA database as reported by EPA (data from U.S. EPA 2002b). | |||||
Risk Characterization
The probability of adverse effects to individual members of a population is calculated as the ratio of the estimated environmental concentration to the relevant toxicological endpoint (LC50 or NOEC) for the most sensitive acute or chronic endpoint. EPA calls the resulting calculation the Risk Quotient (RQ). The lower the magnitude of the RQ, the more likely that no harm would occur from exposure to atrazine in the environment. For acute toxicity of pesticides classified for general use, the EPA uses the LC50, and is unconcerned if the ratio is 0.5 or less. However, if the pesticide has a restricted use classification or if endangered species need protection in the geographical area under scrutiny, then the acceptable RQs would be 0.1 and 0.05, respectively. For chronic toxicity, the NOEC is used, and the estimated environmental concentration should be equal to or less than the NOEC (i.e., the RQ should be less than or equal to 1.0).
If death of amphibians by atrazine exposure were of concern, then the RQ would range from 0.002 to 0.0003 depending on whether the EPA modeling data or the 95th percentile USGS NAWQA monitoring data were used (Table 3, below). Similarly, RQs for tadpole malformations would also be significantly below 0.01 and thus far below EPA’s RQ of concern (0.5). In contrast to the RQs based on endpoints of lethality and malformations, RQs based on sex organ development (i.e., intersex) indicate a high risk for hazard from exposure to modeled atrazine EECs as well as actually measured residues at the 95th percentile for all stream samples (Table 3, below).
Applicability to the Pacific Northwest
The NAWQA combined database for all studied watersheds in the United States tends to skew the percentile of detection to the higher levels of atrazine because of the comparatively high concentrations detected in the watersheds of the Corn Belt, where atrazine is heavily applied. Thus, examining data from individual watersheds may cast more light on regional differences in risk.
Using USGS data from a watershed where atrazine is hardly used, the Willamette basin, the 90th percentile detection of atrazine (0.5 µg/L) would yield an exceedingly high RQ (0.5/0.03=16.7) (Rinella and Janet 1998). On the other hand, atrazine concentrations in the Puget Sound NAWQA study unit were less than 0.025 µg/L, suggesting little chronic risk (RQ = 0.025/0.03 = 0.8) but still above levels of concern for consideration of acute toxicity to endangered species (RQ is 16x the acceptable risk level).
In the EPA’s ecological risk assessment, the agency noted the reports of endocrine system effects on frogs, but chose not to use them for risk characterization (EPA 2002b). EPA traditionally has used the most sensitive fish species and aquatic invertebrate (usually Daphnia spp.) tested to determine aquatic ecological risk. The toxicological endpoint commonly used is the NOEC based on a life cycle test that examines reproductive success, rather than a statistical examination of the gonads. Nevertheless, the exercise in risk calculations illustrated in Table 3 shows how perception of risk changes as the toxicological endpoint changes. Thus, for frog lethality or malformations effects, atrazine would not be considered risky (all RQs > 0.05). However, EPA’s EECs exceed all other endpoints, including invertebrate lethality, reduction in phytoplankton primary production, and intersex condition in frogs. Using the NAWQA database, invertebrate lethality is below EPA’s level of concern but phytoplankton productivity and frog intersex exceed the benchmark RQ of 0.2 for a restricted use pesticide.
TABLE 3 |
||||
| Toxicological Endpoint | EPA
Modeling of EEC Residues
|
NAWQA
Pesticide Residue Data
|
||
| 4
day average |
90
day average |
50th
Percentile |
95th
Percentile |
|
| Lethality (LC50, 10700 µg/L) | 0.002
|
0.002
|
0.000003
|
0.0003
|
| Tadpole Malformations (NOEC, 2600 mg/L) | 0.008 |
0.007 |
0.00001 |
0.0013 |
| Invertebrate Lethality (LC50, 23 µg/L) | 0.909 |
0.817 |
0.001 |
0.141 |
| Reduction in Phytoplankton Primary Production (2.16 µg/L) | 9.68 |
8.7 |
0.013 |
1.5 |
| Intersex (NOEC, est. 0.03 µg/L) | 697
|
626
|
0.9
|
108
|
| Ecological risk characterization of atrazine based on calculations of risk quotients (RQs) using lethality (frogs and invertebrates), phytoplankton primary productivity as a surrogate for phytotoxicity, and endocrine effects (intersex) as toxicological endpoints. Exposure was based on the residue concentrations derived from EPA computer modeling (corn, low rate) and the USGS NAWQA database (agricultural basins) (based on U.S. EPA 2002b). | ||||
Reality Checks
Once a risk characterization is completed, it is a good time to take a reality check to determine if field observations are consistent with the armchair-driven risk assessment outcome. If a pesticide is characterized as being particularly risky, one would expect that an extensive history of actual use in the field should have yielded noticeable ecological effects.
Few field studies have reported the occurrence of intersex adult frogs. One study of ponds in New Hampshire reported two intersex individuals out of over 1400 frogs examined, an approximate 0.14% incidence (Sower et al. 2000). No information was given about possible atrazine contamination in that study. A study in Illinois reported an intersex incidence rate of 2.7% for cricket frog (Acris crepitans) collections during 1993-1995 (Reeder et al. 1998). This incidence rate represented very few individuals and was not significantly correlated with the occurrence of atrazine in ponds. Pertinently, the concentrations in the water bodies ranged from ~1-15 µg/L.
The oft-cited American leopard frogs were sampled from eight aquatic habitats across the United States (Hayes et al. 2002b,c). The sites were chosen on the basis of very low atrazine usage or intense usage. Atrazine was found at all sites, and seven of the sites had frogs with abnormal testicular development. Curiously, the abnormality most frequently found in this field study was testicular oogenesis (presence of eggs in the testicles), while the prevalent effect found in controlled laboratory tests was gonadal dysgenesis (malformed or infertile testes). The occurrence of hermaphroditic frogs tended to range from 10% to 35% with no consistent pattern in relation to atrazine concentrations. However, a site without agricultural impacts and with low atrazine concentrations yielded a whopping 90% of the frogs with gonadal abnormalities. Although the study did not examine reproductive capability, it did pointedly observe that frogs were very abundant at all the localities sampled. Thus, hermaphroditism was observed in the field, but the phenomenon seems without ecological relevance so far.
Based on very limited field observations, intersex may or may not be common, but the frequency seems unrelated to atrazine concentration. However infrequent intersex frogs are, they are not an unnatural occurrence. Examinations of museum specimens of cricket frogs revealed that preserved individuals archived long before the advent of atrazine exhibited a low rate of intersex individuals (V. Beasley, University of Illinois, personal communication, Oct. 17, 2002).
EPA Always Has the Last Word
The agrichemical manufacturing industry has garnered the reputation that it can push the EPA around when EPA makes its decisions about registering chemicals. However, any registration specialist at any chemical company will tell you that EPA definitely has the upper hand. In the Registration Eligibility Decision (RED) documents about atrazine ecological risk, EPA concluded that atrazine was a particularly risky chemical for aquatic life (U.S. EPA 2002b). However, EPA discounted fish and aquatic invertebrates toxicity data and used a NOEC for effects on aquatic vegetation that was in the realm of endocrine effects on frogs. Thus, using a NOEC of 2.3 µg/L and the distribution of NAWQA data, its own modeled EECs, and a probabilistic analysis of how often water concentrations would exceed the endpoint, EPA concluded that atrazine posed a high risk for ecological hazards (see Table 3 for an example of the risk characterization).
Six years ago, when the Food Quality Protection Act was passed, pundits predicted the demise of many pesticides owing to excessive exposure to residues in food and water. For nearly all compounds still registered, dietary and drinking water concerns have evaporated as better data on residues and consumption have been used. However, the Achilles’ heel for many pesticides has shifted to ecological risks, a realm where many are not passing EPA’s litmus test.
Atrazine is certainly in water everywhere, but its fate rests solely on EPA’s decisions, which at this point are based on assessment of ecological hazard and risk. EPA’s revised RED for ecological risk of atrazine, which was issued April 2002, will probably undergo slight changes before EPA offers its final decision on re-registration. All who worry that EPA gives in easily to the wishes of chemical companies should remember that registration isn’t over until the EPA sings.
Dr. Allan Felsot is an Environmental Toxicologist with Washington State University’s Food and Environmental Quality Laboratory. He is a frequent contributor to AENews and can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
REFERENCES
Alford, R. A. and
S. J. Richards. 1999. Global amphibian declines: a problem in applied
ecology. Annual Review Ecology Systematics 30:133-165.
Allran, J. W. and W. H. Karasov. 2001. Effects of atrazine on embryos,
larvae, and adults of anuran amphibians. Environ. Tox. Chem. 20(4):769-775.
Berger, L., R. Speare, P. Daszak, D. E. Green, A. A. Cunningham, C. L.
Goggin, R. Slocombe, M. A. Ragan, A. Hyatt, K. R. McDonald, H. B. Hines,
K. R. Lips, G. Marantelli, and H. Parkes. 1998. Chytridiomycosis causes
amphibian mortality associated with population declines in the rain forests
of Australia and Central America. Proc. Natl. Acad. Sci. 95:9031-9036.
Canfield, J. T., S. M. Kersten, and P. Vanselow. 2000. 1997-1999 Field
season report: Minnesota malformed frog investigation. Minnesota Pollution
Control Agency, Minneapolis, MN. 75 pp.
Carey, C. and C. J. Bryant. 1995. Possible interrelations among environmental
toxicants, amphibian development, and decline of amphibian populations.
Environ. Health Perspectives 103(suppl. 4):13-18.
Carey, C. 2000. Infectious disease and worldwide declines of amphibian
populations, with comments on emerging diseases in coral reef organisms
and in humans. Environ. Health Perspectives 108(suppl. 1):143-150.
Carr, J. A., L. Du Preez, J. P. Giesy, T. S. Gross, R. J. Kendall, E.
E. Smith, K. R. Solomon, and G. Van Der Kraak. 2002. Comments on the paper
entitled "Atrazine-induced hermaphroditism at 0.1 ppb in American
Leopard frogs (Rana pipens): laboratory and field evidence"
by Hayes, T., Haston, K., Tsui, M., Hoang, A. Haeffele, C., and Vonk,
A. Environmental Health Perspectives. Atrazine Endocrine Ecological Risk
Assessment Pane, Ecorisk, Inc., Ferndale, Washington. http://www.cgfi.org/;
link to "Frog Sex-Change Claims Flawed, Could Hurt Farmers and the
Environment," Oct. 30, 2002, CGFI.
Crain, D. A., L. J. Jr. Guillette, A. A. Rooney, and D. B. Pickford. 1997.
Alterations in steroidogenesis in alligators (Alligator mississipiensis)
exposed naturally and experimentally to environmental contaminants. Environ.
Health Perspectives 105(5):528-533.
Garry, V. F., D. Schreinemachers, M. E. Harkins, and J. Griffith. 1996.
Pesticide applicators, biocides, and birth defects in rural Minnesota.
Environ. Health Perspectives 104(4):394-399.
Hayes, T. B., A. Collins, M. Lee, M. Mendoza, N. Noriega, A. A. Stuart,
and A. Vonk. 2002a. Hermaphroditic, demasculinized frogs after exposure
to the herbicide atrazine at low ecologically relevant doses. Proc. National
Acad. Sci. 99(8):5476-5480.
Hayes, T., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2002b.
Feminization of male frogs in the wild. Nature 419(31 October):895-896.
Hayes, T., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2002c.
Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs
(Rana pipiens): laboratory and field evidence. Environmental
Health Perspectives. http://dx.doi.org/,
enter doi: 10.1289/ehp.5932.
Howe, G. E., R. Gillis, and R. C. Mowbray. 1998. Effect of chemical synergy
and larval stage on the toxicity of atrazine and alachlor to amphibian
larvae. Environ. Toxicol. Chem. 17(3):519-525.
Johnson, P. T. J., K. B. Lunde, E. G. Ritchie, and A. E. Launer. 1999.
The effect of trematode infection on amphibian limb development and survivorship.
Science 284:802-904.
Johnson, P. T. J., K. B. Lunde, R. W. Haight, J. Bowerman, and A. R. Blaustein.
2001. Ribeiroia ondatrae (Trematoda: Digenea) infection induces
severe limb malformations in western toads (Bufo boreas). Can.
J. Zool. 79:370-379.
Johnson, P. T. J., K. B. Lunde, E. M. Thurman, E. G. Ritchie, S. N. Wray,
D. R. Sutherland, J. M. Kapper, T. J. Frest, J. Bowerman, and A. R. Blaustein.
2002. Parasite (Ribeiroia ondatrae) infection linked to amphibian
malformation in the western United States. Ecological Monographs 72(2):151-168.
Kavlock, R. J. 1998. What's happening to our frogs? Environ. Health Perspectives
106(12):773-774.
Kiesecker, J. M. 2002. Synergism between trematode infection and pesticide
exposure: a link to amphibian limb deformities in nature? Proc. Natl.
Acad. Sci. 99(15):9900-9904.
Larson, S. J., R. J. Gilliom, and P. D. Capel. 1999. Pesticides in streams
of the United States: Initial results from the National Water Quality
Assessment Program. U.S. Geological Survey Water-Resources Investigation
Report 98-4222, Sacramento, CA :99 pp. http://ca.water.usgs.gov/pnsp/rep/wrir984222/index.html.
Ouellet, M., J. Bonin, J. Rodrigue, J. L. DesGranges, and S. Lair. 1997.
Hind limb deformities (ectromeilia, ectrodactyly) in free-living anurans
from agricultural habitats. J. Wildlife Diseases 33:95-104.
Reeder, A. L., G. L. Foley, D. K. Nichols, L. G. Hansen, B. Wikoff, S.
Faeh, J. Eisold, M. B. Wheeler, R. Warner, J. E. Murphy, and V. R. Beasley.
1998. Forms and prevalence of intersexuality and effects of environmental
contaminants on sexuality in cricket frogs (Acris crepitans).
Environ. Health Perspectives 106(5):261-266.
Rinella, F. A. and M. L. Janet. 1998. Seasonal and spatial variability
of nutrients and pesticides in streams of the Willamette Basin, Oregon,
1993-95. Resources Investigations Report 97-4082-C, U.S. Geological Survey
Water, Portland, OR http://oregon.usgs.gov/projs_dir/pn366/nawqa.html
Sanderson, J. T., R. J. Letcher, M. Heneweer, J. P. Giesy, and M. van
den Berg. 2001. Effects of chloro-s-triazine herbicides and metabolites
on aromatase activity in various human cell lines and on vitellogenin
production in male carp hepatocytes. Environ. Health Perspectives 109:1027-1031.
Sanderson, J. T., J. Boerma, G. W. A. Lansbergen, and M. van den Berg.
2002. Induction and inhibition of aromatase (CYP19) activity by various
classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicology
and Applied Pharmacology 182:44-54.
Sower, S. A., K. L. Reed, and K. J. Babitt. 2000. Limb malformations and
abnormal sex hormone concentrations in frogs. Environ. Health Perspectives
108(11):1085-1090.
U.S. EPA. 2002a. EFED review of comments from Syngenta and its contractors
about the EPA revised environmental risk assessment for atrazine. http://www.epa.gov/oppsrrd1/reregistration/atrazine/,
link to "atrazine."
U.S. EPA. 2002b. Reregistration eligibility science chapter for atrazine
environmental fate and effects chapter. http://www.epa.gov/oppsrrd1/reregistration/atrazine/,
link to "atrazine."
Young, B. E. and 13 others. 2001. Population declines and priorities for
amphibian conservation in Latin America. Conservation Biology 15(5):1213-1223.
Go to this issue's Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page
Announcements & Upcoming Conferences
Items in this section often appear in the words of the sponsoring organization or original news release. AENews editorial staff is not responsible for the accuracy of the content.
Mosquito Control Workshops
The Washington Departments of Agriculture, Ecology, and Health are offering workshops statewide in January to introduce local government and private pest control operators to mosquito control. The training is focused toward those interested in getting started in mosquito abatement in response to West Nile Virus arriving in Washington.
The one-day sessions run from 9:00 AM to 4:00 PM and are planned for the following cities and dates:
| Longview | 7-Jan | County Conference Center, 1942 First Avenue (SE of the Hall of Justice) |
| Tacoma | 9-Jan | Tacoma-Pierce County Health Department Auditorium, 3629 South D Street |
| Mt Vernon | 10-Jan | Skagit County PUD Aqua Room, 1415 Freeway Drive |
| Spokane | 14-Jan | Spokane County Agricultural Education Bldg, 222 N. Havana |
| Wenatchee | 16-Jan | Chelan County Auditorium, 400 Douglas Street |
| Yakima | 17-Jan | Ecology Central Regional Office, 15 W. Yakima Avenue |
| Walla Walla | 22-Jan | City/County Health Bldg, 310 W. Poplar (5th & Poplar) |
Topics will cover the history of mosquito-borne disease in the state, West Nile virus, mosquito vectors and habitat, surveillance, licenses, permits, control materials and their uses, monitoring requirements and record keeping
Who should attend? Local government managers and pest control operators new to or interested in mosquito control requirements and methods or those who are considering starting mosquito control activities and programs.
For registration or additional information contact Kathleen Emmett, Department of Ecology, (360) 407-6478, email kemm461@ecy.wa.gov; Tom Gibbs, Department of Health, (360) 236-3060, email tom.gibbs@doh.wa.gov; or Wendy Sue Wheeler, Department of Agriculture (360) 902-1972, email WSWheeler@agr.wa.gov.
Environmental, Fishing Groups Seek Court Action to Limit Pesticide Pollution
Groups File for Injunction to Protect Salmon from Harmful Pesticides
(Reprinted directly from 11-27-02 news release.)
Environmental and fishing groups have filed for an injunction in Seattle Federal District Court to limit the pesticide uses most likely to harm salmon. The move follows a July court ruling that forces the U.S. Environmental Protection Agency (EPA) to ensure that it does not allow pesticide uses that harm endangered salmon. The groups are seeking the injunction to put interim protections in place until EPA brings its pesticide regulations into compliance with the Endangered Species Act."We know these pesticides are in our rivers and streams and they can harm salmon," said Aimee Code of the Northwest Coalition for Alternatives to Pesticides. "We need the court to put salmon protections in place today."The groups filing for the injunction include the Northwest Coalition for Alternatives to Pesticides, the Washington Toxics Coalition, the Pacific Coast Federation of Fishermen's Associations, and the Institute for Fisheries Resources. They are represented by Earthjustice.
The groups are seeking the following interim protections:
-
a 100-yard no-spray zone to protect salmon from aerial applications of pesticides near salmon streams;
-
a 20-yard no-spray zone to protect salmon from ground applications of pesticides near salmon streams; and
-
a ban on homeowner (non-licensed) use in urban areas of certain pesticides likely to harm salmon.
"There is no time to lose in taking action to get pesticides out of our waterways where they are harming salmon," said Erika Schreder of the Washington Toxics Coalition. "We're asking for common-sense protections to reduce pesticide contamination of our waters while EPA complies with the law."
In July, Judge Coughenour ordered the Environmental Protection Agency to initiate consultations with the National Marine Fisheries Service on protection of salmon from 54 pesticides. These consultations mark the first step toward ensuring pesticide use will not wipe out threatened and endangered salmon.
Water monitoring by the U.S. Geological Survey has found extensive evidence of pesticides in salmon waters, including fourteen pesticides at levels likely to cause harm. The original lawsuit, decided in July, also cited EPA's own documents finding that current uses for numerous pesticides are likely to threaten fish or their habitat. In total, EPA's findings and the U.S. Geological Survey detections identified 54 pesticides that pose documented threats to salmon.The no-spray buffers sought in today's injunction filing would apply to the 54 pesticides in the July court order, and the urban restrictions would apply to products containing 13 selected pesticides that pose particular hazards in urban areas.
The groups are asking the court to put the measures in place while EPA complies with last summer's order. "Our salmon populations are in decline, and we need swift action to address the causes of that decline," said Glen Spain of the Pacific Coast Federation of Fishermen's Associations. "This is a step toward restoring salmon that could bring back tens of thousands of fishing jobs and a billion dollar industry to our region."
"The government has dragged its feet when it comes to protecting salmon from pesticides," said Patti Goldman of Earthjustice. "It's time to limit pesticide contamination of salmon streams until pesticide use is brought into compliance with the Endangered Species Act."
The injunction brief is available at http://www.earthjustice.org.
Washington Toxics Coalition is a non-profit organization in Seattle, Washington, dedicated to protecting public health and the environment by preventing pollution. Northwest Coalition for Alternatives to Pesticides, a non-profit organization in Eugene, Oregon, works to protect people and the environment by advancing healthy solutions to pest problems. Pacific Coast Federation of Fishermen's Associations is the West Coast's largest trade organization of commercial-fishing families, representing thousands of family commercial-fishing businesses and boat owners from San Diego to Alaska. Institute for Fisheries Resources is a marine resource and salmon-conservation organization dedicated to the protection of watersheds and public marine resources. Earthjustice is a non-profit organization providing free legal services to groups that need to go to court to protect the environment.
For further information, contact Aimee Code, NCAP, 541-344-5044 ext.27, Erika Schreder, Washington Toxics Coalition, 206-632-1545 ext.19, or Patti Goldman, Earthjustice, 206-343-7340 ext.32.
Direct seeding (planting and fertilizing directly into the previous crop's stubble in one or two passes without prior tillage) can provide a "win-win" opportunity for agriculture, the public, and the environment. In addition to effective control of cropland soil erosion by wind and water, it offers the potential for reduced production costs and improved profitability for the grower, improved water storage that can result in a higher potential yields, improved soil quality and productivity, and reduced potential of global warming through sequestration of atmospheric carbon in soil with minimal soil disturbance. Many of our global grain market competitors, such as Canada, Australia, Brazil, and Argentina now have 40 to 80% of their cropland under direct seeding. Although the Pacific Northwest is behind their lead, a number of Inland Northwest counties already have over 30% of their cropland under direct seeding. We are beginning a major retooling of agriculture in this region and worldwide with the transition to direct seeding systems and the potential for more intensive cropping.
The Sixth Northwest Direct Seed Cropping Systems Conference and Trade Show will be held January 8-10, 2003, at the WestCoast Hotel in Pasco. The show offers Northwest growers and everyone involved in agriculture an excellent opportunity to learn about the latest technologies and experiences to improve the success of direct seed systems in this region. Check out the Website (http://pnwsteep.wsu.edu/directseed) for all the details, including program agenda, speaker photos and profiles, poster exhibition form, sponsorship and trade show prospectus, pre-registration (with the low $50 fee; $60 at the door), hotel room reservation (starting at an amazing $48 Conference rate), and much more.
http://pnwsteep.wsu.edu/directseed
The conference program features 28 speakers, including 6 growers from Idaho, Oregon, Washington, South Dakota, and New Zealand. Highlights include the great debate on high- versus low-disturbance direct seed openers, stacked rotation and other pest management strategies, transition economics, crop marketing strategies for direct seeders, residue management options, new weed control strategies, managing for increased soil carbon and productivity, and grower experiences across the region. Activities and accomplishments of Pacific Northwest Direct Seed Association (PNDSA) will also be highlighted. At least 10 drill company representatives will present new innovations in direct seed drills and openers for seed and fertilizer placement, residue management and hillside stability. Pesticide applicator and CCA credits will be available for the program.
Over 800 Northwest growers and ag support personnel are expected to attend this conference. It is being organized as a service to growers by two groups: the PNW STEEP program and the grower-driven PNDSA. STEEP (Solutions to Environmental and Economic Problems) is a cooperative research and educational program on conservation tillage systems through the University of Idaho, Oregon State University, Washington State University, and USDA Agricultural Research Service. The conference is being co-sponsored by a number of ag support companies and groups, and developed in cooperation with a dozen PNW grower organizations and ag support groups and agencies.
A special pre-conference feature is that the PNW STEEP 2002 Research Review will be held January 7-8 at the conference hotel. The Review looks at conservation tillage systems research projects underway through the STEEP program in Idaho, Oregon and Washington and attendance is free of charge to conference participants.
This year's Pacific Northwest Pesticide Issues Conference focuses on the issue of Agricultural Safety and Health. The conference will be held at the Yakima Doubletree Hotel on February 26, 2003, and offers recertification credit for Washington, Oregon, Idaho, and Montana.Conference goals are to meet the education and information needs of people involved in farming, forestry, greenhouse, and nursery health and safety, with a strong emphasis on pesticide issues, and to facilitate neteworking and information exchange among participants. Attendees will likely represent grower associations, individual growers, forestry concerns, agrichemical dealers, crop consultants, chemical companies, state and federal regulatory agencies, and university research and extension programs.
The conference is co-sponsored by WSU Cooperative Extension, U of W Pacific Northwest Agricultural Safety and Health Center, U of W School of Public Health and Community Medicine, and the Northwest Center for Occupational Health and Safety. Conference organizer Carol Ramsay can be reached at (509) 335-9222 or ramsay@wsu.edu. More information, including the latest on the conference agenda, can be found at
The Fourth National IPM Symposium/Workshop is an exciting opportunity to learn about the latest developments in agricultural and urban IPM and to share your IPM experiences with others. The symposium includes over 60 breakout sessions (workshop, debate and presentation formats) encompassing almost all aspects of IPM, as well as plenary speakers talking about their experiences in building alliances. In addition, several IPM-related organizations are convening their meetings before or after the Symposium making this a full week of IPM in Indianapolis.
Examples of sessions at the Symposium include:
-
Building Alliances Between IPM Practitioners and Consumers
-
Connecting with the Media/Press
-
Biorational Insecticides - Selectivity and Importance in IPM Programs
-
Federal Agencies and IPM: Improving Communication and Coordination
-
School IPM
-
New Tools for Agricultural Professionals - WeedSOFT: A New Approach in Integrated Weed
-
IPM for Teachers & School Students: K-12 Curricula
-
Barriers to the Adoption of Biocontrol Agents and Biological Pesticides
-
Integrated Crop Management of Greenhouse and Floricultural Crops
-
IPM and Urban Wildlife Pest Situations
-
Application and Prioritization of IPM Projects in Natural Areas
-
Images of Sustainable Agriculture: Landscapes, Pest Management and Biotechnology
-
The Future of Global IPM
-
Landscapes and Weeds
-
IPM in Organic Systems
CALL FOR POSTERS
Share Your IPM Experiences! Do you want to tell colleagues about your IPM work? Research results, information about your IPM organization, and IPM success stories or challenges are all appropriate for poster presentations. Poster submissions will be grouped according to the topic areas listed on the conference web site. Posters will be displayed at specially designated times during the Symposium, and in an atmosphere conducive to informal viewing and discussion. Participants are strongly encouraged to make poster presentations as oral presentations are limited to invited plenary session speakers and organized breakout sessions. Online submission is now available on the symposium Web site. Submission deadline is December 16.For more information, visit http://www.conted.uiuc.edu/ipm
Carrie Foss and Carol Ramsay have updated the Web site for the 2002-2003 Pesticide Education Training season. The Web site has dates, locations, agendas, and registration forms. Point your Internet browser toand select "Recertification and Pre-License Training." Each licensed pesticide applicator in Washington State should receive a hard copy of this information in the mail by the second week in October. Printed copies of the information are also available through county Cooperative Extension offices. For those waiting to hear from WSDA regarding their recertification status, those reports should be in the mail in early November.
Washington State University (WSU) provides pre-license and recertification training for pesticide applicators. Pre-license training provides information useful in taking the licensing exam. Recertification (continuing education) is one of two methods to maintain licensing. (The other is retesting every five years.)
Course registration (including study materials) is $35 per day if postmarked 14 days prior to the first day of the program you will be attending. Otherwise, registration is $50 per day. These fees do not include Washington State Department of Agriculture (WSDA) licence fees. For WSDA testing sites, schedule, or other testing information, call 1-877-301-4555.For more detailed information about WSU's pesticide applicator training, call the Pesticide Education Program at (509) 335-2830 or visit the Web site.
Mosquito Meeting to Address West Nile Virus
The American Mosquito Control Association (AMCA) will hold its annual meeting on March 2-6, 2002, in Minneapolis, Minnesota. The agenda will include major sessions on West Nile Virus. For more information on this meeting and other mosquito control topics, including a searchable database on West Nile Virus information, see the AMCA Website |
 |
Go to this issue's Table of Contents
Go to Agrichemical and Environmental News Index
Go to WSPRS (Washington State Pest Management Resource Service) Home Page

