


To see this report in Portable Document
Format (PDF), please click on the button. ![]() Should
you not have Adobe Acrobat Reader (required to read PDF files),
this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Should
you not have Adobe Acrobat Reader (required to read PDF files),
this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
Open Forum: In an attempt to promote free and open discussion of issues, The Agrichemical and Environmental News encourages letters and articles with differing views. To discuss submission of an article, please contact Dr. Allan Felsot at (509) 372-7365 or afelsot@tricity.wsu.edu; Dr. Catherine Daniels at (509) 372-7495 or cdaniels@tricity.wsu.edu; Dr. Doug Walsh at (509) 786-2226 or dwalsh@tricity.wsu.edu; or Dr. Vincent Hebert at (509) 372-7393 or vhebert@tricity.wsu.edu. The newsletter is available in a hardcopy version for a $15 yearly subscription fee. Please contact newsletter editor Sally O'Neal Coates at (509) 372-7378 or scoates@tricity.wsu.edu for details.
Return to Agrichemical
and Environmental News Index
If ubiquity on hardware store shelves is any indication of product popularity, then homeowners love Roundup (formulated glyphosate). The ready-to-use formulation has such a low toxicity and hazard for eye and skin irritation that it's hard to believe it can injure anything. Yet, squirt it on young plants growing in the cracks and crevices of your driveway and sidewalk, and you will not have any weed problems for the rest of the summer. Owing to glyphosate's systemic abilities, it readily moves from the leaves to all parts of the plant, effectively eliminating any regrowth. But glyphosate's effectiveness is deceptive, giving some people the mistaken impression that it will kill every plant it comes in contact with. The truth is, older plants are much less susceptible to the effects of glyphosate than younger plants, and certain plants like mature woody brush may not be effectively controlled. And once glyphosate hits the ground, it tightly binds to soil, and its phytotoxic capabilities disappear.
But the myth of glyphosate as a macho, kill-anything herbicide has crossed paths with the concerns over the planting of millions of acres of Roundup Ready (RR) canola, corn, cotton, and soybeans. One fear, expressed particularly in the United Kingdom, is that glyphosate is so effective at weed control, that all forage plants used by songbirds will disappear in the wake of mass plantings of RR canola.
Meanwhile, the concern expressed most in North America is about glyphosate-susceptible "wimpy" weeds evolving into glyphosate-resistant "super" weeds. In crop rotations, the glyphosate-resistant volunteers will be uncontrollable and will run wild over the susceptible rotational crop. Perhaps songbirds will thrive, but those "uncontrollable super weeds" and resistant volunteer plants will devastate crop production, not to mention out-compete and therefore supplant the native vegetation.
And there are other concerns about herbicide-resistant crops. In a nutshell, they can be boiled down to five broad hypotheses:
Of course, these and other concerns regarding glyphosate use and Roundup Ready crops have already been reviewed through agencies including the United States Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) and the U.S. Environmental Protection Agency (EPA). Nevertheless, the concerns have not died, and will only be voiced louder as crops resistant to other herbicides enter production. RR crops, having become a huge commercial success, are the guinea pigs for examining the possible ecological threats of transgenic plant protection technology.
In Part 1 of this series ("Squaring Up Roundup Ready Crops," AENews No. 173, Sept. 2000), I discussed the basics of transgenic engineering; in Part 2 ("Giddy 'bout Glyphosate," AENews No. 175, Nov. 2000), I looked at the fears surrounding the use of glyphosate itself. In this essay, I will concentrate on dispelling the myth of "super weeds," addressing the second (resistance), third (gene flow), and fourth (volunteer crops) items above. I will also advocate the need for integrated weed management whether or not herbicide-tolerant transgenic crops are cultivated.
Over the last five years the planting of herbicide-tolerant crops has rapidly expanded to over 75 million acres worldwide (13). As of 1998, herbicide-tolerant transgenic crops were grown on 18, 26, and 44% of U.S. corn, cotton, and soybean acreage, respectively (6). In Canada, over 70% of canola acreage is now planted with herbicide-tolerant varieties (some transgenic, some nontransgenic). But herbicide-resistant weeds had become a big problem long before the advent of transgenic crop technology.
In the world today, about 235 weed biotypes have developed resistance to one or more herbicides; about 80 resistant biotypes have been found in the United States (9). About 22 biotypes have been documented as herbicide-resistant in Washington State. To date, none of the reported incidences of resistant weeds is related to the introduction of a herbicide-resistant crop, whether transgenic or not.
Like insects, weeds can develop resistance when continually selected by a single herbicide or group of herbicides having the same mechanism of toxic action. Weeds develop resistance in one of two ways. First, a few individuals in a population may possess a gene that enhances metabolic detoxification reactions, thereby breaking down the herbicide fast enough to avoid its phytotoxicity. The second and more prevalent method is the occurrence of some individuals with a gene that alters the herbicide's biochemical target site, making the plant resistant to injury. In either case, if these infrequent individuals escape control and successfully go to seed, comparatively more of these resistant individuals will occur in the population during the next growing cycle. Eventually, most of the population will be resistant to the herbicide.
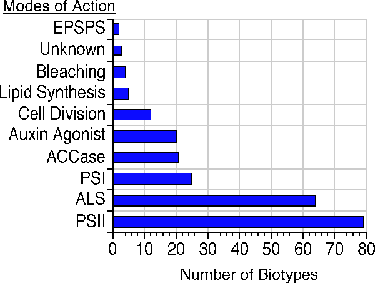
Most cases of herbicide resistance are caused by a gene that produces an insensitive target site. Usually, that target site is an enzyme. The vast majority of weed resistance has developed to herbicides that specifically inhibit the synthesis of amino acids or photosynthesis (Figure 1). For example, herbicides that inhibit the enzyme acetolactate synthase (ALS) kill plants by shutting down branched-chain amino acid synthesis. Sulfonylurea and imidazolinone herbicides are two distinctly different chemical classes that inhibit ALS. Animals lack ALS and the ability to synthesize leucine, isoleucine, and valine (the branched-chain amino acids), so ALS-inhibiting herbicides are of very low hazard to animals. Sixty-three biotypes of weeds worldwide have developed resistance to the effects of ALS inhibitors. The triazine herbicides are one chemical class of several that can inhibit photosynthesis at a specific reaction center, photosystem II, which resides in the plant cell chloroplast, the chlorophyll-containing organelles that make a plant green. Seventy-eight weed biotypes have developed resistance to herbicides inhibiting photosystem II.
|
|
|
|
 |
|
|
Since the overwhelming majority of the herbicide-tolerant crops worldwide are RR, most concerns focus on glyphosate. After 26 years of commercial use, glyphosate resistance has only been documented for two weed species, annual rigid ryegrass (Lolium rigidum) and goosegrass (Eleusine indica) (7, 8). Glyphosate-resistant ryegrass has been confirmed in Australia and California (wheat production), and resistant goosegrass was observed in Malaysia (oil palm production). In both cases, resistance occurred after 10-15 years of intensive glyphosate use (>2 applications per site per season). RR crops, especially soybeans, have been planted on vast acreage for five years now, and no reports of glyphosate-resistant weeds have surfaced.
In addition to direct selection of resistant weeds by repeated application of herbicides with the same mechanism of action, some scientists have hypothesized that transgenes (modified genes) might move from the resistant crop to weeds. Termed "gene flow," the movement of genes between closely related plant species is quite natural and has been occurring ever since flowering plants evolved (14). Gene flow occurs when pollen from one plant species fertilizes flowers of a different but compatible species. The compatible species is usually closely related to the first species. (Gene flow can also occur between populations of the same species.) The offspring resulting from the mating, called hybrids, may then mate via pollen exchange with the wild-type (original) plants. This subsequent mating is called backcrossing or introgression. Introgression causes the hybrid in subsequent generations to become more like the wild-type plant in character and genetic makeup, but it also results in the cultivated plant's genes being stably incorporated into the genome of the wild-type plant. Naturally, a crop plant can receive pollen from a wild-type plant, but chances for successful establishment of this hybrid is poor considering most field crops are grown from certified seed.
A number of cultivated crops, including canola, sugar beet, and wheat, are grown in close proximity to their related wild-type ancestors (14). A few cases of gene flow have been observed under field conditions. On the other hand, corn, soybean, and cotton have no closely related compatible wild-type relatives in the United States or Canada (14). Thus, the likelihood of gene flow from these crops to noncrop species is nil.
Perhaps the most studied examples of gene flow have been in sugar beet (Beta vulgaris) and in canola (Brassica napus). Both of these crops are plagued by the presence of related weedy wild-type plants that cannot be differentially controlled by a herbicide. In France, adventitious beet weeds were hypothesized to be hybrids resulting from pollen transfer between nonweedy wild-type beets and cultivated seed production sugar beets (1). Control of the adventitious beets could be accomplished by introduction of herbicide-tolerant cultivated sugar beets. However, the hybrid weed problem must also be managed by ensuring that seed production sugar beets are not grown in areas where the wild type exists.
Canola (originally a marketing name registered by the Western Canadian Oilseed Crushers Association for food-quality rapeseed oil) was developed from a cross of two wild-type ancestors, Brassica campestris and B. oleracea (11). B. campestris is a common weed in rape production and is not easily controlled with herbicides. Controlled field experiments and genetic analysis of field-collected B. campestris showed that high levels of hybridization occurred between B. campestris and B. napus. Backcrossing (introgression) of B. campestris with itself stabilized the genes of B. napus in the hybrids. But gene flow also occurred from B. campestris to B. napus. Such gene flow possibilities are quite natural, given the close relationship between cultivated oilseed rape and its wild, weedy relative.
Given the ease with which genes can be exchanged between crop plants and their wild relatives (which are weeds in some cases) concern would naturally surface over the potential exchange of herbicide-resistant traits. In such an event, the weedy relatives have been perceived as uncontrollable. Furthermore, there is concern that a transgene will somehow endow a weed with even more aggressive traits (i.e., a "weedier" weed) such as increased seed production. For example, some believe that Johnson grass, a Corn Belt weed, became more fit after hybridization with corn (14).
Ironically, specialists in sugar beet and canola production have eagerly awaited the introduction of herbicide-tolerant cultivars that would enable susceptible weedy relatives to be easily controlled in the same field (1, 11). (See EDITOR'S NOTE; this paragraph originally contained an error.) In Canada, four systems of herbicide-tolerant canola have been approved and commercially introduced. No herbicide-resistant weedy relatives have been reported where these tolerant cultivars are grown.
Controlled experiments, however, have proved that herbicide resistance traits can flow to weedy relatives. Whether the trait will be stable over many generations in the weed is still unknown. A laboratory experiment was used to determine the ability of glufosinate (Basta herbicide) resistant transgenic B. napus to transfer its resistance character (known as the bar gene) to five related species in the family Brassicae (12). While the gene was detected in a number of hybrids, it wasn't always expressed, especially when the mating of the wild type was with the cultivated parent having multiple copies of the bar gene. The gene may have integrated in a place on the chromosomes (i.e., the genome) where gene expression was suppressed.
In another experiment, rape with Basta resistance was crossed with wild radish (Raphanus raphanistrum) (2). The resulting hybrid seeds were then planted in a field of wild radish and studied for four generations. Predictably, the bar gene showed up in half of the hybrids. After four generations of introgression, the chromosome number and morphology of the hybrids were similar to the wild-type radish, but the frequency of Basta resistance was reduced by one half. Given the optimal conditions for pollen transfer in this experiment (i.e., hybrids located in a solid planting of wild radish), the authors of the study concluded that the occurrence of transgene introgression under actual field conditions is probably rare and would occur very slowly.
Few experiments to date have measured flow of herbicide resistance traits between closely related species. Nevertheless, the National Academy of Sciences supports making predictions of the likelihood and consequences of commercial scale, crop-to-wild gene flow from pest-protected plants on the basis of general ecological and agricultural knowledge (14).
Factors affecting the successful establishment of hybrids resulting from gene flow have been reviewed and serve as a useful guide for regulatory questions (3, 14). The likelihood of gene flow between crops and weeds depends on the coexistence of the crop and wild relatives within a distance pollen may travel; on the simultaneous timing of flowering of the various species and their mating compatibility; on the survival and reproductive ability of the hybrids; and on the fate of the genes in wild populations (3). The greater the distance pollen travels, the more likely a wild-type compatible plant will be fertilized. For certified seed production, therefore, definitive distances are set by the USDA to isolate seed crops from potential cross-pollination with different varieties (14). Similar buffer zones could be imposed if transgenic gene flow was determined to be a significant threat.
Immigration of susceptibility genes into a weed population is also possible. In any case, for a hybrid to become established and crop genes be introgressed into the wild-type weed, gene flow of susceptible or resistant genes must be associated with some fitness advantage. In the absence of herbicide selection or other fitness trait such as increased fertility, there is no reason that a weedy wild-type relative outside of the cultivated field would present a problem.
Many crops are rotated annually with unrelated crops. Prior to planting the rotational crop, the field is cultivated to prepare a new seed bed and/or treated with herbicide either before or after emergence of the new crop. This combination of treatments is likely to kill any volunteer crops, herbicide-tolerant or not. However, in fields with little or no tillage, concern has been expressed that the transgenic crop might not be easily controlled, especially if effective herbicide choices were limited by the resistance trait. Furthermore, the crop might become a "super weed" if it "escapes" from the field and establishes on uncultivated land.
The premise for these concerns is that somehow the transgenic character endows the crop with the characteristics of a weed. However, this is a specious argument because herbicide tolerance is conferred by a single gene coding for an altered form of a target site that already exists in the plant. Furthermore, many crops naturally tolerate certain herbicides quite well, yet those crops do not turn into uncontrollable "super weeds."
Prolific seed production is one characteristic of weediness. Increased reproduction of RR crops has not occurred, according to studies showing that yields of transgenic and native cultivars are not significantly different (4). Even if volunteers of rotational crops need to be controlled, there are still many herbicides available with a different mode of action than the one to which the subject plant possesses tolerance. For example, canola in Canada is resistant to three herbicides acting at the biochemical target sites of ALS, EPSPS, or photosystem II. But volunteer canola can still be controlled by 2,4-D (5).
Finally, few crops survive outside of the cultivated field. "Escapees" of corn, cotton, or soybean are unlikely to survive in the wild (14). Canola is a bit different, since it is actually a hybrid of a closely related wild species; it could perhaps survive on uncultivated land. But the ability to survive intact with a herbicide resistance gene doesn't give canola any fitness advantage in the absence of herbicide applications. Outside a cultivated field, it would lose its "incentive" to retain the gene.
Until recently, environmental advocacy groups (EAGs) seemed about as interested in herbicide-resistant weeds as they were in insecticide-resistant insect pests. With the commercial introduction of herbicide-resistant crops, EAGs seem to have found a reason to be intolerant of herbicide-tolerant crops. As discussed earlier, however, resistant weeds have been around a long time. As a result, so have strategies to manage resistance.
With or without transgenic crops, resistance development remains a threat if chemical control is not carefully managed and integrated with nonchemical methods. The Herbicide Resistance Action Committee (HRAC), a consortium of agrichemical manufacturers who provide information about resistance cases and strategies for management, has developed a herbicide risk assessment table to help growers and crop protection specialists determine the probability that certain practices will lead to resistance (Table 1) (10). By conducting weed control using practices listed in the lowest risk category column, growers can minimize herbicide resistance regardless of its cause.
|
|
|||
|
|
|||
|
|
|
|
|
| Herbicide Mix or Rotation |
|
|
|
| Weed Control Method |
|
|
|
| Use of Same MOA* Per Season |
|
|
|
| Cropping System |
|
|
|
| Resistance Status to MOA* |
|
|
|
| Weed Infestation |
|
|
|
| Control Over Last 3 Years |
|
|
|
| *MOA=Mode of Action, Mechanism of Action | |||
Integrated weed management relies on the same principles as any other integrated pest management (IPM) strategy:
In summary, weeds have been developing resistance to various herbicides
for quite a few years. Two types of toxicity in particular, ALS
and photosystem II inhibition, seem to be the biggest problems.
Only two weed species have developed glyphosate resistance after
a quarter century of commercial use. Nevertheless, weed resistance
due to widespread cultivation of herbicide-tolerant crops (whether
transgenic or not) may develop as a result of direct selection
pressure from the herbicide or as a result of gene flow. Gene
flow among closely related species occurs naturally; however,
gene flow from corn, cotton, and soybean to weedy relatives will
not occur in North America owing to the absence of the wild-type
species. Even with species like canola that are cultivated in
proximity to their wild ancestors, hybridization and introgression
of herbicide-resistant genes under natural field conditions seems
to be of low probability. Even if herbicide-resistant genes do
"escape" and establish in weeds, implementation of integrated
weed management can ensure long-term effective control of these
species.
Dr. Allan S. Felsot can be reached at (509) 372-7365 or afelsot@tricity.wsu.edu.
Washington State University offers PRE-LICENSE courses (for those who do not have a license and need one) and RECERTIFICATION courses (for those who need to renew their current licenses). Fees are $35 per day if postmarked 14 days before the program, otherwise $50 per day. This fee DOES NOT include WSDA license test fee, which ranges from $25 to $170; for information on testing and fees, contact WSDA at (360) 902-2020 or http://www.wa.gov/agr/test/pmd/licensing/index.htm. Recertification courses offer 6 credits per day.
The fifth annual Pacific Northwest Pesticide Issues Conference was held on the campus of Pacific Lutheran University in Tacoma on Thursday, October 19, 2000. The annual conference is sponsored by the Washington State University (WSU) Cooperative Extension's Pesticide Education Program. Each year, it focuses on a topic of current interest to the pesticide user community in Washington State. This year's emphasis was the education of and issues facing home and garden pesticide users.
As always, conference co-chair Carol Ramsay packed a lot of information into the one-day event, featuring seventeen speakers in under eight hours. The first hour and a half was allocated to outlining the problems associated with home and garden pesticide use, and the balance of the day focused on solutions.
Bill Mason, Public Health Advisor with the Washington State Department of Health Pesticide Program, began with a presentation addressing human health issues resulting from home and garden pesticide use and misuse. Pesticide-related illness was reported in all but nine Washington counties between 1996 and 1998, with the Puget Sound area accounting for 72% of the illnesses. Generally speaking, about half of reported pesticide exposures are agriculture-related. Of the non-agriculture half, home and garden applications comprise roughly a third of the reports (36% of non-ag, 17% of total 19961998). Mason's statistics also pointed out the wide gap between reported/suspected illnesses and confirmed illnesses. Noting that over twice as many confirmed pesticide illnesses occur as the result of homeowner misuse than a result of licensed pesticide applicator use, homeowner use is an important component of non-ag use. Most home and garden pesticide use resulting in illness is due to negligence in reading labels and subsequent lack of recommended personal protection, product overuse, and inadequate ventilation. Accidental spills and ingestion also factor into the totals, but errors during application are the most common avenue of exposure.
James Ebbert, Supervisory Hydrologist and Project Chief with the U.S. Geological Survey National Water Quality Assessment (NAWQA), spoke about water quality. Reporting on recent studies by NAWQA, Washington State Department of Ecology, and King County, he concluded that pesticides applied by homeowners do indeed contribute to the occurrence of residues in urban streams. Just as certainly, they account for only a portion, as almost half of the twenty-three pesticides detected were not sold in large home improvement stores. These studies found that concentrations of pesticides in ground and surface waters usually did not exceed regulatory safety standard for drinking water.
Jeff Britt, Nursery and Greenhouse Pesticide Specialist for the Washington State Department of Agriculture, discussed the legal implications of home and garden pesticide misuse. About 10% of pesticide law violations stem from residential misuse; about 70 official investigations into homeowner misuse took place in Washington State in 1999. Countless more complaints never turn into investigations, and the majority of violations never even turn into complaints. Homeowner pesticide misuse generally stems from ignorance rather than maliciousness (although a few examples of neighbor-to-neighbor "chemical warfare" were presented, including amusing "hidden camera" tactics!) Homeowners tend to be lax about use of personal protection, dosage, frequency of application, identification of target pest, and disposal, either from misreading or not reading the label, or because they don't understand or appreciate the consequences of violating the label directions.
Some of our region's weightiest urban pesticide issues were addressed in the balance of the morning session.
WSU Extension Entomologist Dr. Art Antonelli and Extension Turf Specialist Dr. Gwen Stahnke tackled the European crane fly. Specifically, what do we do with the loss of Dursban? (See related article, "Turfgrass Clippings" in AENews No. 174, Oct. 2000.) Both European crane fly, which made its first appearance in Washington State in 1966, and the common crane fly, new to the Northwest in 1998, are troublesome pests in western Washington turf. They are undeterred by cold and favored by damp conditions, though the common crane fly can tolerate a drier climate. They are also very difficult to monitor. With Dursban--the preferred crane fly control to date--being removed from the market and no longer available after December 2001, researchers are evaluating numerous chemical, biological, monitoring, and application strategies. Carbaryl (Sevin) and Orthene may be viable options, but only if used after dark with all weed flowers mowed off the lawn, due to bee kill implications. Diazinon is not recommended due to bird and bee lethality. Ethoprop and bendiocarb may have commercial turf utility, but are not likely to be recommended for homeowners.
WSU's Dr. John Stark then provided an ecotoxicologist's perspective on some of the current and potential alternatives to major commercial chemistries. Highlighting recent and current research (much of which uses tiny Daphnia crustaceans as bioassay subjects), Dr. Stark gave an overview of work on a wide range of products: Actara and imidacloprid (neonicotinoids with nicotine's mode of action, but less toxicity); Aphistar and Fulfill (selective aphicides); diazinon; chlorpyrifos; neem (a natural product with several insecticidal properties and little or no non-target toxicity, but also little persistence), Comply (a juvenile hormone for systemic larval control); phloxine B (a photosentitive dye used as a malathion replacement for California med fly); fipronil (a highly toxic but very targeted synthethic); and spinosad (a widely used soft neurotoxin). Research on alternatives considers not only toxicity, targeting, and modes of action, but also sublethal effects, use rates, persistence, and the likelihood of a substance getting into the water.
Amy Breedlove, Program Analyst with the U.S. Environmental Protection Agency (EPA) Office of Pesticide Programs, covered the next two topics: the consumer labeling initiative (CLI) and federal disposal guidance. Research has shown that consumers have a very difficult time understanding pesticide labels. The CLI is a voluntary, cooperative partnership between government agencies and industry to work toward improving the readability of labels. Format recommendations so far include use of more bulleted text, simpler language, more white space, and more tables and graphics where appropriate. Confusion also exists regarding disposal of partly filled pesticide containers. Old recommendations for wrapping the containers in newspaper and discarding in the trash have been discredited since (1) they are rarely followed, and (2) the method doesn't work very well. New recommendations add a toll-free number to labels, remove the "wrap in paper" phrase, and instruct consumers to call their local solid waste agency, since disposal guidelines vary by location. EPA's proposed revised instructions were published in June 2000, and comments were taken through August 2000. A revised draft will be available within the next few months.
Before launching into an afternoon session on the means of properly educating homeowners about safe pesticide use, WSU Agrichemical and Environmental Education Specialist Dr. Doug Walsh treated attendees to a luncheon slide show about the effects of advertising on consumer pesticide choices-a phenomenon that would be humorous if it weren't so true. Much of his talk, though without the amusing visuals, was presented in the article "Pesticide AD-ucation: Homeowners 'Learn' from Madison Avenue," in the October 2000 issue of AENews (No. 174).
 The
first presentation after lunch showed conference attendees that
horticultural concepts and environmental stewardship can be taught
at a young age. Dr. Kerry Richards combined her experience as
a K-12 educator with her doctoral work on agrichemicals to develop
a K-12 curriculum in integrated pest management (IPM) that has
been widely adopted in Pennsylvania schools. Dr. Richards currently
works as Senior Extension Associate and Pesticide Education Coordinator
at Pennsylvania State University. Some of her K-12 educational
materials can be viewed on the Internet at http://www.pested.psu.edu. (At right: "D.
B. Pest," on-line anti-hero for the K-12 set, courtesy of
PennState Pesticide Education Program.)
The
first presentation after lunch showed conference attendees that
horticultural concepts and environmental stewardship can be taught
at a young age. Dr. Kerry Richards combined her experience as
a K-12 educator with her doctoral work on agrichemicals to develop
a K-12 curriculum in integrated pest management (IPM) that has
been widely adopted in Pennsylvania schools. Dr. Richards currently
works as Senior Extension Associate and Pesticide Education Coordinator
at Pennsylvania State University. Some of her K-12 educational
materials can be viewed on the Internet at http://www.pested.psu.edu. (At right: "D.
B. Pest," on-line anti-hero for the K-12 set, courtesy of
PennState Pesticide Education Program.)
Next, WSU Spokane Horticulture Extension Agent Tonie Fitzgerald spoke about the Master Gardener Volunteer Program. Emphasizing the program's professionalism and University support, she explained that it is "more than a horticulture class or a garden club--it is a volunteer program that enables participants to serve their communities through horticultural education." First, the Master Gardener candidates themselves are trained in identification and diagnosis of plant problems and management practices, then they volunteer, transmitting their knowledge to the community via telephone, on-line, and in-person education programs.
An unlikely partnership between Seattle Tilth, Washington Toxics Coalition, WSU Cooperative Extension, and King County Hazardous Waste Management has resulted in an educational effort called the Green Gardening program. Mary Robson of Cooperative Extension explained that the program, now in its eighth year, emphasizes appropriate plant selection and least-toxic pest management strategies to promote IPM and reduce pesticides in the waste stream. The Green Garden program has produced fact sheets on resistant plants, IPM principles, and alternative pest and disease controls. They also present workshops to retail nursery staffs, garden groups, and other interested parties.
Washington State Department of Agriculture (WSDA) Water Quality Protection Manager Cindy Moore spoke next about an educational program piloted in California called "H2O Home to Ocean." Designed to educate homeowners about the impact of improper pesticide disposal, the H2O Home to Ocean campaign was administered through publicly owned wastewater treatment facilities. Personnel shifts caused the California program to fold before its success could be evaluated, but WSDA is investigating whether such a program could help Washingtonians learn about the purchase, use, and disposal of pesticides.
Carrie Foss, conference co-chair and Education Associate with WSU's Pesticide Education Program, provided conference participants with a list of books, websites, and other resources for safe and effective home and garden information. She stressed the importance of using university-approved sources, and proceeded to engage the audience with a slide show of website (mis-)information by self-styled gardening "experts." The audience of university and extension personnel and industry professionals rallied to debunk the outrageously erroneous "recommendations."
The National Pesticide Telecommunications Network (NPTN) is a cooperative effort of Oregon State University (OSU) and the EPA to provide science-based information on pesticides free to the public. OSU Faculty Research Assistant Christa Marie Chadwick filled in for Extension Toxicologist Dr. Terry Miller, Director of NPTN, by providing an overview of NPTN's many functions. The toll-free telephone line at (800) 858-7378 is staffed by trained specialists 6:30 a.m. to 4:30 p.m. Pacific time seven days a week, excluding holidays, and the website is available at http://ace.orst.edu/info/nptn/index.html. NPTN provides chemical, health, and environmental information about more than 600 pesticide active ingredients in over 50,000 different U.S. products.
The final sessions of the afternoon focused on various facets of an issue those in the agrichemical business can no longer ignore: how much should domestic (home and garden) pesticide use be regulated? The question sparks outrage in consumers and weariness among regulators. In a society where you can pick up a handgun at a flea market, does it make sense to license or otherwise regulate homeowners who choose to use pesticides around their property? Do the dangers posed by pesticides merit exercising caution to the extent of governmental interference?
When one hears Amy Breedlove talk about the label misinterpretations EPA uncovered in their CLI research, reads the kind of information Carrie Foss showed conference attendees from the Ed Hume and Jerry Baker websites, or fields a few calls at the WSU Pesticide Information Center (see related article, "The Hazards of Harriet," AENews No. 174, Oct. 2000), the argument for regulation grows stronger. No one wants to see G-men cruising neighborhoods in search of Roundup violations, but something must be done to protect people from themselves--not to mention from charlatans trying to make a buck on fraudulent or questionable product safety and efficacy claims.
Dr. Catherine Daniels, Pesticide Coordinator at Washington State University, made a case for taking another look at those "minimum risk" pesticides exempted from EPA registration under Federal Insecticide Fungicide and Rodenticide Act (FIFRA) Section 25(b). While the intent of Section 25(b) was to reduce the cost and regulatory burden on businesses and the hassle for the public of reading labels on a "safe" product, 25(b) has inadvertently provided a major-league loophole and led to vast and potentially dangerous confusion among consumers. If manu-facturers who invoked the 25(b) exemption truly met all criteria, there would not be a problem, but viola-tions are rampant, both in the spirit and the letter of the regulation. The American Association of Pesticide Safety Educators (AAPSE) and the Association of American Pesticide Control Officials (AAPCO) have both sent requests to EPA to reconsider the necessity of 25(b) labels. WSDA proposed changes in state law this year that would add labeling requirements for 25(b) products registered in Washington (see "Changes for Washington's Section 18 Process" on opposite page.)
WSU Pesticide Education Specialist and conference co-chair Carol Ramsay continued the unpopular theme of domestic pesticide regulation by posing some tough questions about certification and training for home and garden pesticide applicators. Should home and garden pesticide users receive training? If so, should they be tested? Should home and garden retail stores (a significant source of consumer information) require training of their floor staff? Should someone at each retail store receive certification? Should a revised classification system be implemented covering domestic users and dealers? As a pesticide applicator training professional and member of the national Certification Training and Assessment Group (CTAG), Carol is interested in receiving input from stakeholders on this issue at ramsay@wsu.edu. CTAG meets in December 2000.
Ann Wick, Program Manager with WSDA, made the final presentation of the day. She began by acknowledging the importance of the two major themes that had emerged from the conference: (1) the importance of public education and (2) the problems with misleading marketing and pseudo-information. She then proceeded to explain that WSDA views EPA regulations as a baseline, and that WSDA's mission is to protect the public via legislative and regulatory action. Where home and garden users are concerned, WSDA looks to limit the possibility of misapplication via whatever means they deem appropriate, from education to prescribing homeowner formulations (such as exclusively ready-to-use mixtures) to limiting application to licensed applicators.
The Pacific Northwest Pesticide Issues Conference is held annually. Each year focuses on an issue of importance to pesticide users and educators in the Northwest. Watch the WSU Pesticide Education Program web page at http://pep.wsu.edu for information about next fall's conference. A limited number of proceedings from the 2000 conference are still available for $10.00 apiece; contact 2000 conference co-chair Carol Ramsay at (509) 375-9222 or e-mail ramsay@wsu.edu.
WHO: Pesticide Registrants, Applicators, and Distributors.
WHAT: Washington State Department of Agriculture (WSDA) is now requiring that pesticides distributed under a Section 18 emergency exemption be accompanied by a label. The Section 18 label must be reviewed and approved by WSDA and must include all of the conditions for pesticide use, whether they stem from the Section 18 request or the granting document. In addition, a provision has been made for situations where the label cannot be developed and approved prior to the intended use period.
WHEN: The new rules (found in WAC 16-228-1400, Pesticide Labeling Requirements) became effective November 30, 2000.
WHERE: Throughout Washington State.
WHY: Under the old system, when EPA drafted a Section 18 granting document, they may or may not have included all of the specific requirements that WSDA had provided for in the Section 18 request document. Applicators were still held responsible for all of the requirements whether or not they were included in the granting document or in the Section 18 request. While the granting documents are generally distributed, the request documents are not.
With this rule change, WSDA is also adopting specific labeling requirements for Section 24(c) Special Local Need registrations, spray adjuvants, and Section 25(b) minimum risk pesticides. These requirements were adopted to be consistent with current federal and/or state requirements.
A complete copy of the new rule language
will be available on WSDA's website: http://www.wa.gov/agr/pmd/etc/laws.htm.
Draft Risk Assessment Available |
In an ongoing effort to force a job offer from the Environmental Protection Agency (EPA), Her Royal Highness The Queen Bee of Labels (HRH QBL, a.k.a. WSU's Jane M. Thomas) takes time out of her busy Royal Schedule periodically to point out oddities and aggravations on pesticide labels. It is the QBL's Opinion Most High that if she were in charge of all things label, a few RULES, combined with swift and thorough consequences for transgressors, would whip the whole pesticide label business into shape in a matter of weeks. Until such time as EPA sees the light and appoints HRH QBL to her rightful position, The Queen shall content herself with commentary, including nominations for the Non-Anom* Awards, a new industry standard for particularly pathetic, aggrievedly awful, and terribly tacky pesticide labels. For background on this ongoing saga, see "If I Were the Queen of Labels," AENews No. 169, May 2000. For details on the Non-Anoms, see "QBL II," AENews No. 171, July 2000.
 In
last
month's AENews ("Call It Confusing,"
AENews No. 175, Nov. 2000),
readers were promised an example of a case where a pesticide label
was made confusing merely through lousy layout as opposed to vagrant
verbiage. This is all brought about because EPA has still not
confirmed my appointment as the Queen Bee of Labels (QBL), thus
leaving time on the Royal Hands. Always one to use time wisely,
the QBL-elect has been reviewing pesticide labels, gathering ever
more evidence to support the need for some RULES governing the
What and How of pesticide labels and thus the need for a QBL.
Suffice it to say that HRH has now discovered that even the graphic
layout can make a label misleading. This discovery was of an embarrassingly
personal nature, causing the QBL to point out: It's not nice to
fool the Queen.
In
last
month's AENews ("Call It Confusing,"
AENews No. 175, Nov. 2000),
readers were promised an example of a case where a pesticide label
was made confusing merely through lousy layout as opposed to vagrant
verbiage. This is all brought about because EPA has still not
confirmed my appointment as the Queen Bee of Labels (QBL), thus
leaving time on the Royal Hands. Always one to use time wisely,
the QBL-elect has been reviewing pesticide labels, gathering ever
more evidence to support the need for some RULES governing the
What and How of pesticide labels and thus the need for a QBL.
Suffice it to say that HRH has now discovered that even the graphic
layout can make a label misleading. This discovery was of an embarrassingly
personal nature, causing the QBL to point out: It's not nice to
fool the Queen.
Because of the nature of this label indiscretion,
it seems appropriate to introduce a new Non-Anom award category.
Knowing that loyal readers have been all atwitter since last month's
issue, the QBL by royal decree, and without further ado, announces
the Litigious Layouts Non-Anom
category.
Finally, at last, and as promised, the disturbingly graphic presentation
that you have all been waiting for. (You know who you are and
you should be ashamed of yourselves!) The first entrant in the
Litigious Layout category is Laddock S-12. There are two products
of this name registered for use in Washington: One by BASF and
the other by Sipcam Agro. (Note that while the example discussed
below is the Sipcam version of the label, the QBL would ask BASF
to stop smirking because their label, while slightly better, is
no great shakes itself.)
The QBL directs your attention to the actual label excerpt below. (ED. NOTE: A clearer representation of both the original Sipcam label and our "revised" label can be viewed in PDF format.) It appears that the tank mix use directions are given first for corn overall, followed by a subset for use on sweet corn. At least that's how Some initially thought it appeared. After careful study, however, One wondered, if that was the case, then why, under the SWEET CORN: section, were there notes for use on field corn and silage corn. Finally, by ignoring all the visual "clues" (read: "mis-cues"), a clearer understanding of the intent was determined.

The QBL's Graphics Vassal prepared an alternative layout for this same information, showing how the same information could be provided in the same amount of space.

Once appointed to my rightful position as the Queen Bee of Labels there will be a Rule that headers, indentation, capitalization, font size, and so forth may only be used as a means to clarify as opposed to obfuscate pesticide label use directions. While the QBL appreciates boldness in good pesticide labeling, the Font of All Label Opinions would like to underscore the Royal Displeasure at being tripped up by something as basic as label layout. (And speaking of displeasure--really, EPA, how tardy. There is no excuse for this dilly-dallying, unless of course EPA is in the throes of a manual recount.)
Jane M. Thomas can be reached at (509) 372-7493 or jmthomas@tricity.wsu.edu.
It's been said that "for every Ph.D. there is an equal and opposite Ph.D." Those who attended the "Agriculture and Water Quality in the Pacific Northwest" conference in Eugene, Oregon, last month got to see this principle in action. When over sixty-five speakers make presentations over a two-day period about a topic as multi-faceted as water quality, there are bound to be contradictions.
The conference didn't disappoint. The ambitious agenda included an array of keynote speakers, concurrent sessions, panels, and posters "designed to improve communication, build understanding, and foster cooperation between people in agriculture, the environment, and government." Representatives of each of these sectors were among the event's sponsors, and attendees represented a wide range of those concerned with the quality of our water here in the Pacific Northwest.
One of the more controversial bodies of research was presented the first day of the conference by Dr. Nathaniel Scholz of the National Oceanic and Atmospheric Administration/National Marine Fisheries Service (NOAA/NMFS). NMFS, through its Northwest Fisheries Science Center in Seattle, has been investigating the sublethal effects of common pesticides, especially diazinon, on various salmonid species. Much of this research has centered upon the salmon olfactory nervous system-that is, the salmon "nose." This organ is extremely sensitive. Many important salmon behaviors appear to be triggered by olfactory cues, including predator recognition, reproductive activities, and homing ability. Pesticides routinely detected in Northwest waters have been shown, at certain concentrations, to affect salmonid olfactory sensitivity, therefore the implication is that behaviors directly related to species survival can be adversely impacted by pesticide residues.
As Dr. Scholz was presenting his research upstairs in Room C, a concurrent session was underway downstairs in Room B. Speakers at this session included Dr. Lenwood Hall from the University of Maryland. Dr. Hall's presentation stressed the need for multiple lines of evidence in predicting site-specific ecological effects. Without referring to Dr. Scholz' NMFS research specifically, Dr. Hall cautioned against using single-species toxicity tests or other single lines of evidence to indicate ecological impairment. Ecosystems, argued Dr. Hall, are quite different than lab experiments or even single-species/single-line-of-evidence field studies because of the complex inter-relationships in ecosystem elements.
While Drs. Scholz and Hall were not necessarily in disagreement (Dr. Scholz presented his research as preliminary in nature and Dr. Hall allowed that single lines of evidence are valuable as first-tier assessment tools), their presentations illustrated some of the inherent difficulties in today's regulatory climate. Science and policy-making are two very different processes; they require different timetables and different levels of understanding. We live in a fairly open society, where preliminary research like Scholz' is available to average citizens and to policy makers not trained in scientific methodology and unable to make thorough, considered, science-based assessments of information. Most scientists would agree that basing regulations and policy on preliminary research is ill-advised, but citizens can have emotional reactions to preliminary data, and those reactions can motivate political action. And we all know what motivates politicians.
Sensing the potential for a juicy debate, the fearless conference organizers scheduled an impromptu panel discussion during the second day's luncheon. Drs. Scholz and Hall, accompanied by Novartis' Dennis Tierney (Novartis is diazinon's registrant), made brief presentations and responded to questions. It was no surprise that Scholz emphasized once again the preliminary nature of his work, and that Tierney emphasized diazinon's safety record. Hall's presentation was a bit more directly confrontational, critiquing conclusions presented in Scholz' paper "Diazinon Disrupts Antipredator and Homing Behaviors in Chinook Salmon" published earlier this year. He pointed out the wide gap between NMFS' measurable effects levels and real-world monitoring levels (actual pesticide residue concentrations detected in Pacific Northwest waters). Scholz had shown behavioral effects in salmon at 10.0 micrograms per liter (µg/L), marginal results at 1.0 µg/L, and no effects at 0.1 µg/L. Monitoring data has shown only 0.3% of samples to contain more than 1.0 µg/L. About ninety-three percent of samples contain less than 0.1µg/L. While Dr. Hall did not disagree with the significance of Dr. Scholz' research, he disagreed with any presumption of effect at environmentally realistic exposures.
In addition to watching regulatory and academic Ph.D.'s go head-to-head, conference attendees had the opportunity to see the directors of Washington, Oregon, and Idaho's departments of agriculture appear side-by-side in a panel discussion. The contrast between Oregon Director Phil Ward's optimism and Washington Director Jim Jesernig's pessimism was palpable; both were balanced by Idaho Director Pat Takasugi's humor and ease of delivery.
Takasugi led off with an animated and somehow entertaining overview of Idaho's current programs. Justifiably proud of his state's successful Idaho Dairy Program, he explained how over 11,000 waste inspections have taken place in Idaho since 1996, with virtually every dairy in the state under scrutiny for compliance with the Clean Water Act. Violations have decreased over time, and the success of the dairy program has spawned the Idaho Beef Cattle Program, another step toward enhancing the good environmental record of this predominantly Republican state.
Director Takasugi also briefed the audience on work being done at the Federal level by the National Association of State Departments of Agriculture (NASDA). NASDA is working on a cost-of-production safety net, on a program called Ag-Flex (Agriculture Flexibility Partnership Act, based on education's Ed-Flex), and on tax reform. Tax breaks on which NASDA is working include elimination of capital gains tax on estates; a one-time out-of-agriculture "transition" tax exemption; 100% deduction for health insurance; 30% credit for monies donated to certain research and agricultural promotion activities; and elimination of taxation on disaster relief funds.
Despite 1999 statistics showing Oregon's net farm income at its lowest since 1983, Phil Ward managed to present a relentlessly optimistic picture of his state's agricultural future. He told of a recent trip to China where he observed Oregon grass seed in use for erosion control behind a massive dam on the Yangtze, an area where farmers routinely cultivate 25- to 30-percent slopes. He spoke with pride of the seven Agricultural Water Quality Management Area Plans already completed in Oregon, and of twenty more underway and a total of sixty planned.
Jim Jesernig made no attempt to disguise his frustration with the three complex interrelated regulatory concepts threatening sustainable management of agricultural water in Washington State: the Endangered Species Act (ESA), the Clean Water Act, and the Boldt decision, a 1974 judicial act assigning 50% of all harvestable fish to Native Americans. Observers now worry about a "Boldt II" proposition that would extend the responsibility for providing tribal fish harvests to parties affecting fish populations in any way, regardless of proximity to tribal fishing grounds. While Jesernig praised his department's pesticide monitoring and regulatory activities, he cautioned that programs including buffer zone designations and compilation of Field Office Technical Guides (FOTGs) are difficult, complex, and hampered by poor data quantity and quality.
A number of speakers and panels discussed TMDLs, or Total Maximum Daily Loads. A TMDL is a calculation of the maximum amount of a pollutant that a waterbody can receive and still meet water quality standards, and an allocation of that amount to the pollutant's sources. Through the Clean Water Act, the U.S. Environmental Protection Agency (EPA) has the authority to review TMDLs, but states, territories, and tribes set the standards.
In Washington State, 643 bodies of water have been identified as "impaired." Waterbodies in Oregon and Idaho number 1100 and 800, respectively. The states have the responsibility to identify the uses for each waterbody (e.g., drinking water supply, swimming, fishing) and to set TMDLs based upon scientific criteria to support those uses.
TMDLs are subject to public review and EPA review. Components of a TMDL include (1) the sum of the allowable loads of a single pollutant from all contributing point and nonpoint sources, (2) a margin of safety to ensure that the waterbody can be used for the designated purposes, and (3) consideration of seasonal water quality variation. If EPA does not approve a state-submitted TMDL, it has the ability to take over the program in that state.
EPA expects a TMDL to provide the means and mechanisms to achieve the targeted pollutant reductions. Compliance with pollutant reduction efforts at point and nonpoint sources is achieved through a combination of enforcement and voluntary means. Monitoring is a critical component of the program, to show that progress is being made toward the reductions targeted.
Currently, EPA and all the states are working under regulations that went into effect in 1992. Under these regulations, states must submit their revised lists of impaired waterbodies every two years. Different states have established different program goals and methodologies with EPA under the 1992 guidelines. For example, in Washington and Oregon, implementation plans are to be part of the TMDL submittal package, whereas in Idaho, the state has eighteen months after EPA's approval of their TMDLs to submit their implementation plans.
July 13, 2000, a new set of regulations affecting TMDLs was approved. These new rules do not go into effect until October 2001. Basically, the new rules are more specific with respect to certain criteria and timelines. EPA will expect monitoring or modeling plans with milestones, and will require reasonable assurances that a stated implementation plan is underway. The new language also clarifies the criteria for an EPA takeover in the event a state-proposed TMDL is not approved. A new ten-year timeline is established for meeting water quality goals.
Much basic TMDL information was provided by EPA Region 10 Watershed Restoration Unit Manager Christine Pysk. Other speakers presented actual "in the trenches" accounts of TMDL development. One of the more enlightening presentations came from a four-person panel representing the Upper Grande Ronde River Subbasin. Mitch Wolgamott of the Oregon Department of Environmental Quality, Ken Diebel of the Oregon Department of Agriculture, Lyle Kuchenbecker of the Grande Ronde Model Watershed Program (the local watershed council), and Jeff Zakel of the Oregon Department of Fish and Wildlife offered a teamwork perspective on a project that is extremely participatory and collaborative in nature. Their project was the first comprehensive subbasin-scale TMDL completed in Oregon. Beginning in 1997, it took two and a half years wherein the fourteen-member stakeholder committee met monthly and various workgroups met as needed. They learned that good leadership and committed participation on the part of all stakeholder groups is essential, that it was more work than they bargained for, and that it might have been easier to complete the technical work before trying to develop the implementation plan, rather than working on the two simultaneously.
The EPA headquarters' TMDL homepage, with links to specific states, is at http://www.epa.gov/owow/tmdl. The 2000 Agriculture and Water Quality in the Pacific Northwest conference was held Tuesday and Wednesday, October 24 and 25, in Eugene, Oregon. Proceedings and attendee list are available on-line at http://www.agwaterqualitynw.org/. The next conference will be held in 2002, with preliminary details posted on the website in early 2001.
Sally O'Neal Coates, Editor of AENews, can be reached at (509) 372-7378 or scoates@tricity.wsu.edu.
In nature, the carpet beetle is a useful insect. Adults feed on nectar and pollen and may actually aid in cross-pollination of flowers. The larvae are efficient scavengers-part of Nature's clean-up crew. In a home or business, however, carpet beetles can wreak havoc far beyond what their small size would indicate. As the weather turns colder, carpet beetle larvae may move from hidden sites to warmer, more visible areas. Species include the following:
Varied Carpet Beetle
Adults are 1/16- to 1/8-inch long. The body is black with a pattern of yellow and white scales on the pronotum (area directly behind head) and elytra (wing covers). Antennae are short, with clubbed ends in three segments. Larvae are dark brown, stout, and teardrop shaped. Larvae are covered with bands of brown hairs pointing toward the rear.
Furniture Carpet Beetle
Adults are 1/16- to 1/8-inch long. The body is black with a pattern of white, yellow, and brown scales on the pronotum and elytra. The underside of the body is covered with white scales. Antennae are short, with clubbed ends. Larvae are similar to the varied carpet beetle.
Black Carpet Beetle
Adults are 1/8- to 1/4-inch long, and dark brown to black in color. Antennae are short and clubbed. The body is elongated and oval; the head is concealed when viewed from above. Larvae are light to dark brown and carrot-shaped, covered with golden brown to dark brown hairs lying flat along the body. The rear end has a tuft of brown hairs nearly as long as the body.
Carpet beetle larvae can infest a wide variety of materials including hair, fur, wool, feathers, hide, horn, animal carcasses, dead insects, rodent nests, bird nests, wasp nests, cereal, stored grain, nuts, seeds, dried peppers, spices, meal, and flour. Food products infested with varied or furniture carpet beetle larvae should be destroyed as the hairs from the body can cause gastroenteritis if ingested.
To control carpet beetles it is necessary to find the source of the infestation. Some things to look for include
Carefully inspect under appliances (you would be surprised how much food you can find under a stove, refrigerator, or washing machine!) In homes with wool or wool/synthetic blend carpets, check under furniture. (The best bet is under whatever is heaviest and hardest to move, of course!)
The key is to start where larvae are most often seen. The adults do no damage; before and after mating, their presence does not usually indicate the site of infestation. Once the source is found, immediately remove the infested material(s). Vacuum the area thoroughly and immediately remove the vacuum cleaner bag and deposit in the trash outside. In most cases this will solve the problem and no pesticide treatment will be required.
Jack Marlowe is the owner of Eden Advanced Pest Technologies (http://edenpest.com) and current President of Washington State Pest Control Association. He can be reached at (800) 401-9935 or edenapt@olywa.net.
In reviewing the October 2000 postings in the Federal Register, we found the following items that may be of interest to the readers of Agrichemical and Environmental News.
In the October 11 Federal Register EPA announced that the preliminary risk assessments and related documents for dichlorvos (DDVP) were available for review. These documents are available electronically at the following URL: http://www.epa.gov/pesticides/op/ddvp.htm. (Page 60430)
In the October 11 Federal Register EPA announced that the revised version of the pesticide science policy document entitled "The Role of Use-Related Information in Pesticide Risk Assessment and Risk Management" was available. This document is available electronically under "additional papers" at the following URL: http://www.epa.gov/pesticides/trac/science/. (Page 60435)
In the October 17 Federal Register EPA announced that it has developed and is requesting comments (due to EPA by 1/16/01) on a draft technical guidance paper for managing agricultural sources of nonpoint pollution. This guidance is intended to provide technical assistance to state program managers and others on the best available, economically achievable means of reducing nonpoint source pollution of surface and ground water from agriculture. The guidance provides background information about agricultural nonpoint source pollution, where it comes from and how it enters the Nation's waters, discusses the broad concepts of assessing and addressing water quality problems on a watershed level, and presents up-to-date technical information about how to reduce agricultural nonpoint source pollution. An electronic copy of this draft guidance paper is available at the following URL: http://www.epa.gov/owow/nps/agmm/index.html. (Page 61325)
In the October 20 Federal Register EPA announced its preliminary determination to terminate the Special Review for triphenyltin hydroxide (TPTH). In 1985 EPA had decided that a Special Review was necessary based on concerns over developmental toxicity. EPA also cited concerns for reproductive toxicity, carcinogenicity, immunotoxicity, inhalation toxicity and adverse effects to non-target organisms. Since this time, voluntary actions by the registrants have reduced worker exposure to TPTH and additional data has allowed for a refined risk assessment. EPA now believes that the risks of using TPTH are substantially lower than when the Special Review was initiated in 1985 and that the benefits of use outweigh the risks. (Page 63173)
In the October 25 Federal Register EPA established a final rule governing the establishment of time-limited tolerances and exemptions for residues of a pesticide chemical resulting from its emergency use as authorized under FIFRA Section 18. The purpose of this rule is to establish a process that will ensure timely decisions on any tolerance-related issue in response to a request for an emergency use of a pesticide chemical to be used in or on food or feed. These rules, now codified as 40 CFR 1 Part 176: Time-Limited Tolerances for Emergency Exemptions, can be found at the back of the Federal Register notice. (Page 64126)
The PNN is operated by WSU's Pesticide Information Center for the Washington State Commission on Pesticide Registration. The system is designed to distribute pesticide registration and label change information to groups representing Washington's pesticide users. PNN notifications are now available on our web page. To review those sent out in the month two months prior to this issue's date, either access the PNN page via the Pesticide Information Center On-Line (PICOL) Main Page on URL http://picol.cahe.wsu.edu/ or directly via URL http://www.tricity.wsu.edu/~mantone/pl-newpnn.html. We hope that this new electronic format will be useful. Please let us know what you think by submitting comments via e-mail to Jane Thomas at jmthomas@tricity.wsu.edu.
|
|
||||||
| Chemical (type) | Federal Register | Tolerance (ppm) | Commodity (raw) |
|
||
|
|
|
|
||||
| azoxystrobin (fungicide) | 10/17/00 | 25 | Brassica | Yes | New | 12/31/01 |
| pg. 61270 | leafy vegetables | |||||
| Comment: This time-limited tolerance is being established in response to EPA granting a Section 18 exemption for the use of azoxystrobin on leafy vegetables grown in Georgia to control leaf spot. | ||||||
| norflurazon (herbicide) | 10/19/00 | 3 | Bermuda grass hay | Yes | Extension | 11/30/02 |
| pg. 62629 | 2 | Bermuda grass forage | ||||
| Comment: These time-limited tolerances are being extended in response to EPA again granting Section 18s for the use of norflurazon to control annual grass weeds in Bermuda grass in various southern states. | ||||||
| tebuconazole (fungicide) |
10/19/00 pg. 62634 |
2 | barley grain | Yes | Extension | 12/31/01 |
| 20 | barley hay | |||||
| 20 | barley straw | |||||
| 4 | hops | |||||
| 0.4 | sunflower oil | |||||
| 0.2 | sunflower seed | |||||
| 15 | wheat hay | |||||
| 2 | wheat straw | |||||
| Comment: These time-limited tolerances are being extended in response to EPA again granting Section 18s for the following tebuconazole uses: Idaho, Oregon, and Washington for the control of stripe rust in barley; Oregon to control stripe and leaf rust in wheat; Michigan, Minnesota, North Dakota, and South Dakota to control Fusarium head blight in barley and/or wheat; Colorado, Kansas, Nebraska, and North Dakota for the control of rust on sunflower; Idaho, Oregon, and Washington to control powdery mildew on hops. | ||||||
| zinc phosphide (rodenticide) | 10/19/00 | 0.1 | timothy | Yes | Extension | 2/1/03 |
| pg. 62631 | 0.1 | alfalfa | ||||
| 0.1 | clover | |||||
| Comment: These time-limited tolerances are being extended in response to Section 18 requests for the continued use of zinc phosphide to control voles in these crops in Washington, Nevada, and California | ||||||
| flufenacet/thifluamide (herbicide) |
10/27/00 pg. 64363 |
1 | wheat grain | Yes | Extension | 7/31/03 |
| 10 | wheat forage | |||||
| 2 | wheat hay | |||||
| 0.5 | wheat straw | |||||
| 0.05 | meat, kidney, and fat of cattle, goats, horses, hogs, and sheep | |||||
| 0.1 | meat by-products of cattle, goats, horses, hogs, and sheep | |||||
| Comment: These time limited tolerances are being extended in response to Section 18 requests to again use flufenacet to control annual ryegrass in wheat in Oregon, Idaho, and Washington. | ||||||
| azoxystrobin (fungicide) | 10/27/00 | 1 | watercress | Yes | Extension | 10/30/02 |
| pg. 64366 | ||||||
| Comment: This time-limited tolerance is being extended due to continued requests for the use of azoxystrobin to control cercosporsa leaf spot on watercress in various states. | ||||||
W.S.U. Pullman Home Page Comments and questions: cdaniels@tricity.wsu.edu Technical Assistance: scoates@tricity.wsu.edu Copyright © Washington State University / Disclaimer Electronic Publishing and Appropriate Use Policy University Information: 509/335-3564