
May 2002, Issue No. 193
A monthly report on environmental
and pesticide related issues
In This Issue
NOTE
ABOUT PRINTING A HARD COPY
Some AENews feature articles are available
in Portable Document Format (PDF) version, which is recommended for
printing. If you do not have Adobe
Acrobat Reader (required to read PDF files), this free program is
available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
If you choose to print from the HTML version you currently see on
your screen, you may need to set your browser's printing preferences
so that no margins are cropped.
|
||
NOTE
ABOUT PRINTING A HARD COPY
Some AENews feature articles are available
in Portable Document Format (PDF) version, which is recommended for
printing. If you do not have Adobe
Acrobat Reader (required to read PDF files), this free program is
available for download at http://www.adobe.com/prodindex/acrobat/readstep.html
If you choose to print from the HTML version you currently see on
your screen, you may need to set your browser's printing preferences
so that no margins are cropped.
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
Using “Soft” Pesticides to Conserve Natural Enemies in Washington Potato Fields |
Click here for PDF version of this document (recommended for printing). Should you not have Adobe Acrobat Reader (required to read PDF files), this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html |
Dr. William E. Snyder and Amanda Fallahi, Entomologists, WSU
Several major insect pests attack potatoes in the Pacific Northwest, including aphids, spider mites, Colorado potato beetles, and wireworms. Organophosphate and other broad-spectrum pesticides are available to control these pests, but may be lost in the future as the Food Quality Protection Act is implemented. Also, these broad-spectrum pesticides kill not only pests but also beneficial insects and spiders, and so are not compatible with biological control. For these reasons potato growers in Washington are interested in new, more selective (a.k.a. “softer”) pesticides.
Softer pesticides could allow the conservation of native natural enemies in potato fields. Increased densities of predators will slow the rate of pest resurgence following pesticide application, and thus can lead to fewer total pesticide applications in a season. The goal of our research, funded by the Washington State Potato Commission, is to improve biological control in Washington by learning more about how we might conserve beneficial insects through the use of softer pesticides.
Our field work in 2001 had two parts:
- intensive sampling of the predator communities in potato fields treated with soft and hard pesticides, and
- cage experiments where we
reproduced predator communities typical of fields treated with soft
and hard pesticides, and then measured the predators’ impact on
green peach aphids.
Potato Regional IPM Project
The potato regional IPM program is a cooperative effort between university and private researchers, growers, and the Washington State Potato Commission. The regional IPM program is a large-scale comparison of whole potato fields, where some of the fields are treated with hard pesticides and others are treated with newer, more selective pesticides. The “hard” fields were treated with Methamidophos (Monitor) for aphids and Esfenvalerate (Asana) for Colorado potato beetles, while the “soft” fields were treated with Pymetrozine (Fulfill) for aphids and Spinosad (Success) for Colorado potato beetles.
We intensively sampled predator populations in eleven production potato fields that were part of the Regional IPM program. Broad-spectrum pesticides were applied to eight of the fields and selective pesticides were used on the remaining three. As an additional comparison we also sampled predators from three certified organic fields, for a total of fourteen fields sampled.
Using a D-vac machine, which is essentially a giant vacuum cleaner that is powerful enough to suck up insects, we collected predators from 100 randomly selected plants in each field on each of two sample dates. The first sample from each field was collected in July, and the second sample was collected in August. Thus, we had one sample from each field that was collected around the time of canopy closure and a second sample after canopy closure.
We found that four predators were very common: big-eyed bugs, damsel bugs, ground-dwelling dwarf spiders, and a second group of spiders that hunt in the plant canopy. (Big-eyed bugs and damsel bugs have been featured in “Bug of the Month” articles in AENews. See http://www.aenews.wsu.edu/Oct01AENews/Oct01AENews.htm#BugOMonth and http://www.aenews.wsu.edu/Dec01AENews/Dec01AENews.htm#BugOfTheMonth , respectively.) Both big-eyed and nabid bugs were significantly more abundant in the soft pesticide & organic fields than in fields treated with broad-spectrum pesticides (Figure 1). Densities of dwarf spiders did not significantly differ among the three treatments. Spiders other than dwarf spiders were most abundant in organic fields.
FIGURE 1 |
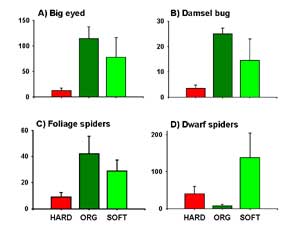 |
| Densities (per 100 plants) of A) big-eyed bugs, B) damsel bugs, C) dwarf spiders and D) other spiders in hard, soft and organic potato fields. 100 plants were sampled in each field at each of two sample dates. Big-eyed and damsel bug densities, and spiders other than dwarf spiders, were most abundant in soft and organic fields and less common in broad-spectrum fields. Dwarf spiders were most abundant in fields treated with soft pesticides. Predators were as much as 10X more abundant in fields treated with soft pesticides, compared to those treated with broad-spectrum pesticides. |
Overall, predator densities were highest in soft and organic fields and very low in fields treated with broad-spectrum pesticides. Soft pesticide and organic fields had similar predator densities. However, the predator composition of the three types of fields also differed (Figure 2A-C). Broad-spectrum fields were dominated by dwarf spiders (Figure 2A), while big-eyed bugs were the most abundant predators in selective pesticide fields (Figure 2B). Organic fields were similar to conventional soft-pesticide fields in community composition, except that big-eyed bugs made up an even larger fraction of the predator community and spiders other than dwarf spiders were relatively common (Figure 2C).
FIGURE 2 |
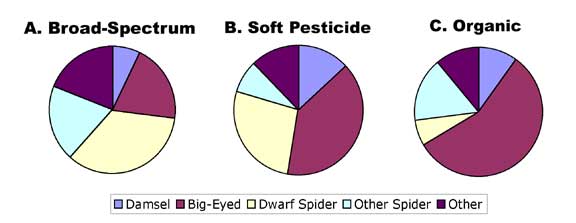 |
|
Proportional
makeup of the generalist predator community in potato fields treated
with A) broad-spectrum pesticides, B) selective pesticides, and
C) certified organic fields. Hard pesticide fields were dominated
by dwarf spiders, while the most abundant predators in selective
and organic fields were big-eyed bugs.
|
Cage Experiments
We conducted a field experiment in which we manipulated natural enemies to determine their impact on green peach aphids. Our field experiments were conducted in a potato field at the WSU Othello Research Unit in Othello, Washington. In this experiment we manipulated predator densities to achieve the following three treatments: 1) predators removed (treatment O), simulating predator densities typical of hard pesticide fields; 2) predators added at the average predator density, determined as the average number of each predator collected in production fields during our field sampling (AVG), simulating typical predator densities in fields treated with soft pesticides; and 3) high predator density added, determined as the mean of the three highest densities recorded during our field sampling (HIGH); simulating predator densities typical of organic fields. After releasing or removing predators, we introduced thirty aphids to each cage. Once a week for three weeks, we monitored aphid densities by carefully hand searching and counting aphids on each potato plant. We found that predators slowed the rate of aphid increase 90% in the two predator treatments (Figure 3).
FIGURE 3 |
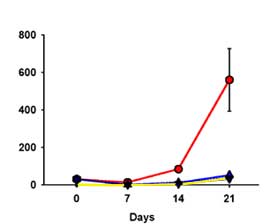 |
| Green peach aphid populations in large field cages with predators removed (red line), average predator densities (light blue line), or with high predator densities (yellow line). Aphids increased very rapidly when predators were removed, but slowly when predators were present. Predators used were at densities typical of fields treated with soft pesticides. |
Summary
The selective pesticides we examined were very effective in conserving predators. Predator densities were as much as ten times higher in fields receiving soft instead of hard pesticides. In most cases predator densities in soft fields were similar to those in organic fields. Our cage experiments demonstrated that predator densities in soft and organic fields are high enough to greatly slow the rate of aphid increase. However, predator densities in fields treated with broad-spectrum pesticides are probably too low to have much impact on aphids. Because predators are very effective at slowing the rate of aphid increase, conserving predators through the use of selective pesticides may allow growers to reduce the total number of sprays needed each year.
Bill Snyder and Amanda Fallahi are with the Department of Entomology at Washington State University in Pullman. They can be reached at wesnyder@wsu.edu or (509) 335-3724.
Return to Table of Contents for the May, 2002 issue
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
Some Corny Ideas About Gene Flow and Biodiversity |
Click here for PDF version of this document (recommended for printing). Should you not have Adobe Acrobat Reader (required to read PDF files), this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html |
Dr. Allan S. Felsot, Environmental Toxicologist, WSU
Mother Nature has been taking a beating. Her products are receiving a bum rap. Carbon dioxide, the gas that plants need to make sugars and that nearly all organisms respire, has been decried as a pollutant amidst fears that it is the principal cause of global warming. The latest hit against Mother Nature’s ways came in the USA Today headline “Gene-altered DNA may be ‘polluting’ corn” (Manning 2001). Behind the headline was a tale from the science weekly, Nature, about genetically engineered snippets of DNA that were found in native varieties of corn grown in Mexico. The DNA was claimed to have flowed into the native corn varieties (or landraces) via pollen from U.S. corn hybrids that contained a gene from the insect pathogen, Bacillus thuringiensis (Bt). The gene was inserted into the genome of the U.S. corn hybrids using the techniques of biotechnology so that the plants would produce a protein that is selectively toxic to specific insect pests, namely the European corn borer and the corn earworm. Such plants can be called biotechnology derived (i.e., BD plants or crops) to distinguish them from plants bred conventionally by laborious crossing and selection of desirable traits over many years.
The DNA in question was called
a pollutant because it shouldn’t have been in the Mexican corn. Bt-corn,
as the genetically modified commodity is called, is not allowed into Mexico.
Perhaps some farmers who wanted to grow more food and make some money
made a mistake out of ignorance. Apparently not, according to the newspaper-quoted
author of the report that appeared in Nature (Quist and Chapela
2001). The principal investigator from the University of California at
Berkeley (UCB) warned, “The probability is high that diversity is
going to be crowded out by these genetic bullies.” Furthermore, the
UCB investigator stated categorically that plants with the Bt toxin have
“been shown to have potentially very bad effects on insects and the
microbes in the soil.”
Stimulated by the Nature paper, environmental advocacy groups (EAGs)
issued yet another proclamation for a total ban on all BD crops. No one
wants to see biodiversity destroyed and soil fertility ruined by “crop
pollution.” A spokesperson for the Union of Concerned Scientists
(UCS) summed up another belief among the EAGs when he said, “We should
not be going forward on an experiment when we have no idea of the parameters”
(Manning 2001).
If carbon dioxide and DNA are considered pollutants, could it be that Mother Nature is meaner than we think? Are we threatening biodiversity and soil health by our complete lack of knowledge of what the heck we are doing? Or are the reports and hand wringing over the UCB investigators’ letter to Nature magazine just one more mischaracterization of what is really going on? What do we really know about the parameters related to biodiversity of corn in its native homeland and the possible impact of BD corn?
Gene Sleuths
First, exactly what was reported in the letter to Nature (Quist and Chapela 2001)? Samples of corn from an isolated region near Oaxaca, Mexico, were collected and analyzed for genetic markers that would indicate the presence of transgenic DNA. Transgenic DNA in this case would be any DNA that is not naturally present in the corn genome but comes from other plant or bacteria species. Specifically, the UCB scientists were looking for either a Bt toxin gene (i.e., a whole gene that codes for a protein known as Cry1Ab) or a snippet of DNA called the cauliflower mosaic virus 35S promoter (CaMV 35S). The CaMV 35S sequence could also come from Roundup Ready corn, a variety that is modified to resist the herbicide glyphosate. CaMV 35S DNA does not code for a protein but rather functions to help BD plants transcribe Bt genes into messenger RNA for eventual translation into proteins. Corn plants don’t normally have genes for Cry1Ab nor DNA for CaMV 35S unless they are introduced by biotechnological methods.
In essence, the UCB scientists were testing the hypothesis that pollen from illegally planted Bt corn had fertilized native Mexican landraces. A landrace is still Zea mays, the name for all corn, but it has been developed in Mexico and adapted to its specific climatic conditions.
The UCB scientists did not say why they suspected that illegal corn hybrids were brought into the country nor did they explain their reason for choosing particular corn samples to test. Nevertheless, the researchers tackled the corn samples with a technique called PCR (polymerase chain reaction) that enables detection of very tiny amounts of DNA by synthesizing many strands from only a single strand (for a lucid explanation of PCR techniques see http://www.accessexcellence.org/AB/IE/PCR_Xeroxing_DNA.html). In other words, one copy of a gene or DNA sequence in one corn seed out of hundreds can be amplified into over a billion copies to identify a DNA sequence that may be derived from genetic engineering.
The UCB scientists concluded they found evidence of CaMV 35S in five of seven landrace corn samples. The conclusion of BD DNA “contamination” was solely based on the use of two consecutive PCRs to detect a piece of the CaMV 35S promoter DNA. In other words, so little CaMV 35S was present in the native landraces that two amplification cycles were required to detect it.
Based on many generations of crossing descendants of the original Bt corn plants, the gene construct containing CaMV 35S is known to be stable. However, the UCB scientists used a technique called inverse PCR to indicate that the CaMV 35S DNA introgressed into the native landrace genome at multiple regions and also broke into smaller fragments. If this random insertion of the promoter DNA or its pieces all over the genome did happen, then it is possible that normal development of the seed could be disrupted. The UCB scientists also reported that one corn sample tested positive for the Bt Cry1Ab toxin gene, but the Nature article did not provide the DNA evidence to prove that the Bt gene was actually present let alone functional.
Considering that small farmers in Mexico select their seed for desirable traits and then replant it (Louette 1997), the UCB scientists implied that the presence of BD DNA threatened the integrity and sustainability of the Mexican corn landraces. Moreover, the UCB scientists stated their concern for “future genetics of the global food system” in the presence of the widespread planting of BD crops. Yet, those concerns did not motivate the UCB team to plant the “rogue” seeds to determine whether the Bt character or the CaMV 35S were stable introgressions and whether the seed was even viable. Such experiments seem a necessary first step to even begin answering the bigger concerns of impacts on biodiversity.
The UCB scientists gained more than just the admiring attention of the media and environmental groups. Their failure to take the next logical step and redo the tests on the next generation of plants from the rogue seed before publication brought the critical attention of molecular biologists from numerous academic and government institutions.
The Gene is Out of the Bottle
Within days after the release of the UCB report, CIMMYT (International Maize and Wheat Improvement Center), a public research foundation whose mission is preservation of maize biodiversity and crop improvement, released a press release of their foundation’s own results in a search for biotechnology-derived DNA introgressions (CIMMYT 2001). None of the forty-three Oaxacan landraces in CIMMYTs gene bank or a new collection of forty-two different varieties had detectable levels of CaMV 35S promoter.
The editorial board of the journal Transgenic Research issued an essay critiquing the UCB report (Christou 2002). Furthermore, two critical letters that were published in Nature uncovered profound shortcomings in the methods and interpretations by the UCB researchers (Kaplinsky et al. 2002; Metz and Futterer 2002). In short, major flaws were found upon critical examination of the experimental design and techniques. PCR tests alone are subject to artifacts (i.e., false positives) and must be confirmed by additional types of molecular tests commonly exercised to confirm PCR results (for a pictorial explanation of the technique see http://www.accessexcellence.org/AB/GG/southBlotg.html). The critics recommended that all claims of introgressed BD DNA should also be supported by growing out the F1 hybrid (i.e., planting the rogue seeds, which are actually the progeny, or F1 generation, that grows into the next generation of plants) and re-doing the molecular tests along with examining obvious effects on plant morphology. Nature allowed the UCB researchers to answer their critics in a rebuttal that included additional data not included in the original report. Nature’s editors encouraged readers to make up their own mind about the “truth.”
The controversy over “DNA pollution” grew as environmental advocacy groups banded together to issue a joint statement denouncing industry-paid, biotechnology proponents in academia and government for personally attacking the integrity of the UCB scientists. In response, a statement was put together with signatures from scientists all over the world stating that critique of research methods are not ad hominem attacks and that science could only progress by constant skeptical inquiry and correction. (The Joint Statement 2002) (As a skeptic, I admit I signed the latter statement).
Such controversy is the stuff movies are made of. Well, at least newspaper headlines. The press was still having a field day months after the story broke. Witness the March 20, 2002 headline and leader in the Christian Science Monitor: “Calling Poirot: bizarre case of cross-border 'super corn'. Scientists claim genetically modified grain from US invades Mexico, threatening purity of birthplace of corn” (Belsie 2002).
The Genes Flow In and the Genes Flow Out
The lead sentence of the Christian Science Monitor headline encapsulates a common misconception about plants, namely that their genome is somehow fixed (i.e., pure). Plants, unlike animals, are immobile and must rely on dispersal of pollen through physical (e.g., wind) and biological (e.g., bees) processes. Without the gene flow that occurs from dispersed pollen, plant populations are likely to go through a genetic bottleneck from too much inbreeding and consequently suffer reduced genetic diversity and possible fitness (Mayr 1971). Indeed, farmers in Mexico have noted reduced productivity after growing local varieties (i.e., landraces) for numerous generations in the same field without the benefit of significant pollination from other varieties (Gonzalez and Goodman 1997). Thus, wind- and insect-pollinated plants are naturally “promiscuous,” and it is for their own good.
Frankly speaking, the idea that Mexican landraces are “pure” is absurd. Let’s set the record straight. With few exceptions, modern food crops are not ancient inviolate species. In essence, they are human-directed inventions of genetic manipulation by educated trial and error coupled with intense selection pressure. Without human intervention our crops would not be here for our use, whether they are U.S.-improved cultivars or Mexican landraces. The very fact that genes could be easily exchanged between our food crops and their ancestors has allowed continuous improvement in agronomic traits. Such an exchange of genes between unlike populations of the same or related species is called hybridization, and it’s perfectly natural, especially in plants (Mayr 1970)
.Somehow the myth was started that introgression of “foreign” genes into native landraces of corn would reduce biodiversity. Ironically, Mexican farmers have long been exchanging seeds from local varieties with each other to improve productivity (and genetic diversity) of their corn (Louette 1997). The difference between Mexican and U.S. seed corn production practices boils down to open pollination vs. hybridization. In the United States, inbred seed lines (i.e., corn varieties that are allowed to pollinate only themselves) are crossed each year to produce superior performing (and more genetically diverse) hybrids. U.S. farmers pay a premium for hybrid corn bought every year from seed companies. Hybrid corn has a certified genetic makeup, and it consistently yields well under the environmental conditions in which it was developed. In Mexico, farmers grow their own seed from varieties that are open-pollinated. In other words, they grow varieties that are subject to cross-pollination (i.e., gene flow) from similar varieties or non-local varieties.
Indeed, studies of grower practices in Mexico show that there are many different distinct varieties of corn grown in fields in close proximity to one another. In the region of Cuzalapa on the western Pacific coast of Mexico, twenty-six distinct varieties were grown in a 24,000-hectare watershed containing 1000 hectares of corn (Louette 1997). Fifty-three percent of the corn in the watershed was produced from an individual farmer’s own seed planted in previous years. The rest of the corn was produced from seed exchanged with other farmers in the same watershed (36%) or from seed outside the region (11%). One of the non-local varieties was identified as an improved cultivar of hybrid corn from the United States.
Because Mexican farmers make no attempt to segregate different varieties, plenty of cross-pollination has been occurring (estimated at 38% probability for outcrossing in the Cuzalapa region) (Louette 1997). About one-third of local corn varieties may already have introgressed genes from non-local and improved varieties (Gonzalez and Goodman 1997). Consequently, a continuum of morphological traits and genetic characteristics exits among all the major local varieties (Louette 1997). In other words, within a region abrupt shifts from one morphological trait to another were absent. Seeds in a given field were not necessarily all one color (white, blue, or yellow); rather, mixtures were present (a.k.a. heterozygosity). Yet, despite the tremendous amount of gene flow from non-local to locally adapted and selected cultivars, the varieties survived intact as recognizable entities.
So what is the problem with biodiversity should a gene derived from biotechnology-based breeding outcross to a local landrace variety? Given that a plethora of genes are moving among distinct local varieties and non-local varieties all the time without loss of biodiversity, the answer seems to be “nothing is wrong” other than some people are hung up about the process of breeding rather than the results. I maintain that the real focus of concern should be the ecological effects of gene flow in the context of the local habitat, not the origin of the DNA.
Teosinte: The Great Granddaddy of All Corn
For a head start on answers to the question of ecological effects of gene flow, we can learn a lot by close examination of the relationship between modern corn and teosinte, a grass recognized as the feral progenitor species of corn that grows in Mexico and some Central American countries (Benz 2001). At one time, teosinte was classified as a separate species, Zea mexicana, but modern genetic analysis indicates it is more likely a subspecies, Zea mays subsp. parviglumis. Thus, if gene flow and introgression were going to have any ecologically significant effects, it would have already happened to teosinte in regions where modern corn and the wild grass are growing near one another.
The stinging critique of the UCB study by the editorial board of Transgenic Research began its argument by stating a long-known reality: wind pollination would inevitably lead to gene flow between domesticated crop varieties and their wild ancestors when grown in close proximity to each other (Christou 2002). Indeed, studies on travel distances of corn pollen (e.g., Table 1) show that the potential for gene flow between corn and teosinte is very high whether the plants are growing together in the same field, the teosinte is growing along the borders of the cornfield, or the teosinte is growing in dense patches outside of the cornfield. In both Europe and the United States, recommended distances for separation of hybrid seed-cornfields are 200 m. Corn pollen can be detected at distances greater than 800 m from a field (Eastham and Sweet 2002).
TABLE 1 |
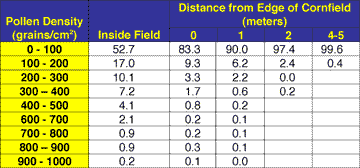 |
| Deposition of pollen (as a percentage falling into each density category) on milkweeds as a function of distance from the edge of a cornfield (modified from Pleasants et al. 2001). Note that few pollen grains are deposited beyond 5 m, although a single pollen grain may travel for over 800 m (Eastham and Sweet 2002). Most of the pollen leaving a field is blocked by the first several rows in a field. Thus, successful pollination between crop varieties would depend on distance of separation between fields and density of pollen flow. In corn, one pollen grain would have to land on the tip of the female flower (called a silk) for successful fertilization and production of one embryo (seed) on a cob that would normally have hundreds of seeds. |
Despite the tremendous potential for gene flow between modern corn and teosinte, the literature about the origin of maize and likelihood of introgressions with teosinte suggest a great deal of uncertainty about whether introgressions are even occurring in the direction of cultivated corn to the teosintes (Doebley 1984; Kato Y. 1997), or whether such introgressions can become fixed without selection pressure (Martinez-Soriano and Leal-Klevezas 2000). For example, if a trait conferring insect resistance in a landrace introgressed into teosinte, that trait would not be important unless the particular pests were also feeding on teosinte and more importantly, were also major mortality factors limiting spread of the plant. Similarly, if herbicide tolerance introgressed into a wild relative, the gene would not be important unless herbicides were used in the areas where the plants are growing.
Flower Power
The big difference between teosinte and corn is in the flower (inflorescence) and seed morphology (Wilkes 1997) (Figure 1). Teosinte has multiple branching inflorescences that only produce two seed rows after fertilization. Modern corn generally produces one large inflorescence but has multiple rows of seeds. Teosinte produces a seed covered in a very hard coat called a glume that is not digestible by animals. The glume of modern corn has been reduced to that white stuff that sticks in your teeth when you take a bite out of sweet corn. Finally, teosinte seeds easily break off the inflorescence and can disperse themselves. Corn seeds do not break off the cob and are incapable of self-dispersal. Given the differences in morphology between teosinte and corn, hybrids should be easy to spot. Indeed, hybrids of corn and teosinte have been found in the field, as well as produced by artificial pollination techniques, but the seeds either do not germinate or the F1 (initial) generation is not very fit (Kermicle 1997). The inability of corn to disperse its own seed also limits its ability to escape from fields and invade teosinte habitat.
FIGURE 1 |
 |
| Evolution in morphology of Zea mays from ancestral teosinte (left) to modern corn (right). The middle figure shows possible hybrids of teosinte and corn landraces. |
The striking evolutionary divergence
in inflorescence morphology of domesticated maize and teosintes exists
to this day, suggesting genetic isolation after the initial characteristics
of consumable corn were fixed despite the known gene flow between the
subspecies. Recent research shows that a gene called tb1 largely controls
the difference in inflorescence morphology. The key to understanding why
teosinte and corn remain morphologically distinct and teosinte is able
to retain its diversity may lie in the function of tb1. Like many genes,
tb1 has a region that actually codes for a protein (the transcribed region)
and a region that is not transcribed but acts like a controller over the
transcribed region (the regulatory region). The regulatory region functions
as a switching area to turn on and off transcription of DNA to messenger
RNA. The transcribed regions of tb1 in both cultivated maize and teosinte
have maintained their polymorphic character (i.e., their genetic variability
or diversity still exists). The non-transcribed regulatory region of tb1
in modern corn, however, has only 3% of the genetic variation found in
teosinte (Wang et al. 1999). Thus, both corn and teosinte maintain their
separate diversity in inflorescence character, which is coded for on the
transcribed region of tb1, and only the control mechanism of modern corn
has been altered over time with loss of its original genetic diversity.
Given the fact that at minimum several hundred years of artificial selection
were required to fix the changes in the regulatory region of tb1, it is
difficult to support a hypothesis that a transgene coding for a pest resistance
character would all of a sudden change biological diversity in teosinte
or native landraces in the absence of intense selection pressure. Indeed,
after thousands of years of cultivation of different varieties of corn
in the presence of teosinte, teosinte still retains diverse forms but
none of them look (or act) like modern corn.
Biodiversity Redux
One of the arguments about the “problem” of BD plants grown near wild relatives is that a transgene could flow to its feral ancestor. The resulting hybrid would acquire a fitness that could elevate it to the status of super weed, crowding out its unfortunate ancestor. The problem with this hypothesis is its focus on the derivation of the gene rather than on the biology of the plants. Crop hybridization with wild relatives has long been known, and in some isolated cases there has been increased weediness of the hybrids (Ellstrand 2001). But the highlighted examples of potential problems involve conventionally bred plants and presumably introgression of numerous genes. We can test whether a new trait (e.g., insect resistance or herbicide tolerance) due to a known single gene will increase fitness. For example, field research from the U.K. has shown that, over a period of ten years, herbicide-resistant corn did not survive well outside of the agricultural field and never took on the characteristics of a weed (Crawley et al. 2001). In recognition of possible advantages in fitness or acquisition of weedy characteristics in introduced plant species, the U.S. regulatory system requires consideration of such events for BD crops (NRC 2002).
Concerns about gene flow and
biodiversity need to focus on specific crop species and ecological situations
on a case-by-case basis. If one is concerned about loss of teosinte, or
even Mexican landraces, whenever BD corn is introduced into Mexico, then
the following question should be considered: if hybridization between
crops with enhanced traits and feral relatives is so rampant and so likely
to reduce biodiversity, why has teosinte remained distinct with its known
diversity of subspecies? After all, distinct landraces of Zea mays
have been grown near teosinte for many hundreds of years. Such deductive
reasoning suggests no effect on biodiversity for corn-teosinte interactions,
but does not absolve responsibilities for careful testing under field
conditions.
A corollary question is: Why have crops with superior qualities for insect
(and/or herbicide tolerance) not become weeds after ten years of testing
(Crawley 2001)? Part of the answer is lack of appropriate selection pressure,
if indeed the hybrids are stable plants. Also, some crop plants themselves
are probably not fit enough or lack characteristics to take on the habits
of weeds. For example, corn seeds do not disperse and therefore are not
likely to become invasive.
On the other hand, certain characters, such as ability to survive drought or salty soils, might impart different selective advantages (Crawley 2001). In that case, those situations should be studied, but the problem is independent of how the characters were bred into the crop. The National Research Council (NRC), the research arm of the National Academy of Sciences, emphasizes that how crops are bred, whether by laborious hand selection and crossings over many years, or quickly by the techniques of molecular biology, is irrelevant to assessing ecological risk (NRC 2000, 2002). The characters produced by the techniques should be the focus of discussion, and they should be assessed in the relevant environments where the crops will be produced.
If we really care about biodiversity, then we should pay attention to efficiency of land use and environmental benefits of crop improvement. Obtaining more yield per acre of land with reduced inputs of pesticides should make more land available for conservation. This goal seems attainable in Mexico where research suggests that all the gene flow over the last half-century between local landraces and non-local varieties, including improved hybrids, has increased per-acre yields (Gonzalez and Goodman 1997) (Table 2).
TABLE 2 |
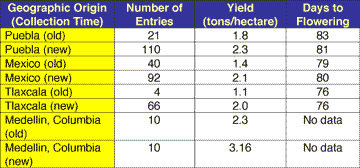 |
| Comparison of yields of different landraces collected approximately 30 years apart in Mexico and in Medellin, Columbia. The data are based on Gonzalez and Goodman (1997), who summarized the research from two independent studies. Seeds collected years ago were stored in germplasm seed banks using practices to ensure the viability of the seed. Periodically the seed is grown out and a new generation of seeds is obtained. Caution should be used in interpreting the data because differences in yield could be due to physiological effects from the age of the seed or it could be due to enhanced vigor associated with hybridization of local landraces with new varieties. Nevertheless, the data suggest that gene flow among landraces has the capability of improving productivity and thus can increase land use efficiency as measured by yield per hectare. |
The problem with biodiversity
does not lie with how crops are bred. Rather, it lies with land management.
A statement from the executive summary of a meeting concerning the impacts
of modern corn on prospects for survival of teosinte sums up our misplaced
concerns about transgenic corn cultivars.
“Changes in land use — especially increased grazing and urbanization
— are the principal threats to teosinte. In recent decades there
has been a drastic reduction in teosinte populations and the danger of
extinction is real. In fact, transgenic maize may be considered a marginal
threat, compared with the effects of urban growth” (Serratos et al.
1997).
Ironically, if teosinte did adapt with a few more weedy characteristics, it would probably fare better as its habitat is reduced in scope. But based on past experience, that doesn’t seem likely to happen.
Dr. Allan Felsot is an Environmental Toxicologist with the Food and Environmental Quality Laboratory on the Tri-Cities campus of Washington State University. Dr. Felsot, a frequent contributor to AENews, can be reached at afelsot@tricity.wsu.edu or (509) 372-7365.
References
Benz, B. F. 2001. Archaeological evidence of teosinte domestication from Guila Naquitz, Oaxaca. Proceedings National Academy of Sciences 98(4):2104-2106.
Belsie, L. 2002. Calling Poirot: bizarre case of cross-border 'super corn'. The Christian Science Monitor, March 20, 2002. http://www.csmonitor.com/2002-0320/p05s01-ussc.html (accessed March 25, 2002).
Christou, P. 2002. No credible scientific evidence is presented to support claims that transgenic DNA was introgresssed into traditional maize landraces in Oaxaca, Mexico. Transgenic Research 11:iii-v.
CIMMYT (International Maize and Wheat Improvement Center). 2001. Further tests at CIMMYT find no presence of promoter associated with transgenes in Mexican landraces in gene bank or from recent field collections. Press Release, December 14, 2001.
Crawley, M. J., S. L. Brown, R. S. Hails, D. D. Kohn, and M. Rees. 2001. Transgenic crops in natural habitats. Nature 409:682-683.
Doebley, J. F. 1984. Maize introgression into teosinte--a reappraisal. Annals of the Missouri Botanical Gardens 71:1100-1113.
Eastham, K. and J. Sweet. 2002. Genetically modified organisms (GMOs): the significance of gene flow through pollen transfer. European Environmental Agency, Copenhagen, Denmark.
Ellstrand, N. C. 2001. When transgenes wander, should we worry? Plant Physiology 125:1543-1545.
Gonzalez, F. C. and M. M. Goodman. 1997. Research on gene flow between improved maize and landraces. In Gene Flow Among Maize Landraces, Improved Maize Varieties, and Teosinte: Implications for Transgenic Maize, CIMMYT, Mexico, D. F. pp. 67-72 (available at http://www.cimmyt.org/ABC/Geneflow/geneflow_pdf_Engl/contents.htm).
Joint Statement in Support of Scientific Discourse in Mexican GM Maize Scandal. 2002. http://www.agbioworld.org/jointstatement.html (accessed March 18, 2002).
Kaplinsky, N., D. Braun, D. Lisch, A. Hay, S. Hake, and M. Freeling. 2002. Maize transgene results in Mexico are artefacts. Nature 416:601.
Kato Y., T. A. 1997. Review of introgression between maize and teosinte. In Gene Flow Among Maize Landraces, Improved Maize Varieties, and Teosinte: Implications for Transgenic Maize. Serratos, J.A., M.C. Willcox, and F. Castillo-Gonzalez (eds.). CIMMYT, Mexico, D.F. pp. 44-53 (available at http://www.cimmyt.org/ABC/Geneflow/geneflow_pdf_Engl/contents.htm).
Kermicle, J. 1997. Cross compatibility within the genus Zea. In Gene Flow Among Maize Landraces, Improved Maize Varieties, and Teosinte: Implications for Transgenic Maize, CIMMYT, Mexico, D. F. pp. 40-43 (available at http://www.cimmyt.org/ABC/Geneflow/geneflow_pdf_Engl/contents.htm).
Louette, D. 1997. Seed exchange among farmers and gene flow among maize varieties in traditional agricultural systems. In Gene Flow Among Maize Landraces, Improved Maize Varieties, and Teosinte: Implications for Transgenic Maize, CIMMYT, Mexico, D. F. pp. 56-66 (available at http://www.cimmyt.org/ABC/Geneflow/geneflow_pdf_Engl/contents.htm).
Manning, A. 2001. Gene-altered DNA may be 'polluting' corn. USA Today, November 29, 2001 :p. 15D.
Martinez-Soriano, J. P. R. and D. S. Leal-Klevezas. 2000. Transgenic maize in Mexico: no need for concern. Science 287(5457):1399.
Mayr, E. 1970. The breakdown of isolating mechanisms (hybridization). Populations, Species, and Evolution. Harvard University Press, Cambridge, MA :Chapter 6, pp. 69-81.
Metz, M., and J. Futterer. 2002. Suspect evidence of transgenic contamination. Nature 416:600-601.
National Research Council (NRC). 2000. Genetically Modified Pest-Protected Plants: Science and Regulation. National Academy Press, Washington, D.C.
National Research Council (NRC). 2002. Environmental Effects on Transgenic Plants. The Scope and Adequacy of Regulation. National Academy Press, Washington, D.C. 320 pp.
Quist, D. and H. I. Chapela. 2001. Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico. Nature 414 (29 November):541-543.
Pleasants, J. M., R. L. Hellmich, G. P. Dively, M. K. Sears, D. E. Stanley-Horn, H. R. Mattila, J. E. Foster, T. L. Clark, and G. D. Jones. 2001. Corn pollen deposition on milkweeds in and near cornfields. Proc. National Academy Sciences 98:11919-11924.
Serratos, J. A., M. C. Willcox, and F. Castillo-Gonzalez, eds. 1997. Executive Summary. In Gene Flow Among Maize Landraces, Improved Maize Varieties, and Teosinte: Implications for Transgenic Maize, CIMMYT, Mexico, D. F. pp. vii-xi (available at http://www.cimmyt.org/ABC/Geneflow/geneflow_pdf_Engl/contents.htm).
Wang, R.-L., A. Stec, J. Hey, L. Lukens, and J. Doebley. 1999. The limits of selection during maize domestication. Nature 398:236-239.
Wilkes, H. G. 1997. Teosinte in Mexico: personal retrospective and assessment. In Gene Flow Among Maize Landraces, Improved Maize Varieties, and Teosinte: Implications for Transgenic Maize, CIMMYT, Mexico, D. F. pp. 10-17 (available at http://www.cimmyt.org/ABC/Geneflow/geneflow_pdf_Engl/contents.htm).
Return to Table of Contents for the May, 2002 issue
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
National Ag Center Aids ComplianceEPA Center Demystifies Environmental Issues |
Click here for PDF version of this document (recommended for printing). Should you not have Adobe Acrobat Reader (required to read PDF files), this free program is available for download at http://www.adobe.com/prodindex/acrobat/readstep.html |
Karrigan Bork, ECO Intern, National Ag Center
 The
National Agriculture Compliance Assistance Center (National Ag Center)
is the first stop for people in the agricultural community who need information
on compliance with environmental regulations. Part of the U.S. Environmental
Protection Agency's (EPA’s) Office of Compliance, the National Ag
Center promotes compliance by helping members of the agriculture community
better understand the environmental requirements that affect their business.
The National Ag Center also provides information to promote the latest
pollution prevention technologies and best management practices.
The
National Agriculture Compliance Assistance Center (National Ag Center)
is the first stop for people in the agricultural community who need information
on compliance with environmental regulations. Part of the U.S. Environmental
Protection Agency's (EPA’s) Office of Compliance, the National Ag
Center promotes compliance by helping members of the agriculture community
better understand the environmental requirements that affect their business.
The National Ag Center also provides information to promote the latest
pollution prevention technologies and best management practices.
Growers, livestock producers, other agribusiness professionals, and agricultural information/education providers can access the Center’s resources easily via telephone, fax, mail, and the Internet (see contact information at the end of this article). The National Ag Center is working with the USDA and other agencies to function as the agricultural community’s definitive source for federal environmental compliance information. The National Ag Center also helps regional and state agencies provide compliance assistance to local agricultural communities.
The National Ag Center offers information on a variety of topics including pesticides, animal waste management, emergency planning, ground/surface water, tanks/containment and solid/hazardous waste.
Why Was the National Ag Center Created?
Many business sectors, including agriculture, are comprised primarily of small businesses. Keeping up with the latest regulation can be difficult, especially for business without personnel dedicated to addressing environmental concerns. The EPA recognizes the desire of these groups to comply with environmental requirements and developed the National Compliance Assistance Centers (http://www.assistancecenters.net) to help the business community comply. In addition to the National Ag Center, the EPA established ten other Centers, serving painting and coating companies, the transportation industry, local governments, printers, metal finishing operations, automotive services and repair businesses, printed wiring board producers, small chemical manufacturers, and federal facilities.
Do the Compliance Assistance Centers Help?
After only a few years of operation, the Compliance Assistance Centers are showing results. The Centers’ Web sites serve a combined total of over 1460 users daily. Online surveys find that users like the services offered by the Centers and that users are improving their environmental performance with the Centers’ assistance. Concerned citizens are taking the initiative to make changes that both benefit the environment and save money. Over 70% of companies and local government users reported changing their practices to improve environmental performance. Of those, 69% believed that they saved money due to their adjustments. The National Ag Center alone has distributed over 43,000 documents to the ag community in the last year. The Centers are working to make compliance an integral part of the small business culture.
With Whom Does the National Ag Center Work?
The National Ag Center has pioneered partnerships with groups throughout the agricultural community. For example, USDA liaisons work directly with the National Ag Center to promote understanding within EPA of the workings of USDA and the agriculture community by participating in National Ag Center and EPA activities. Regular meetings hosted by the National Ag Center feature guest lecturers from the USDA, the United States Geological Survey, and various trade associations, to promote understanding of ag issues among regional and headquarters EPA employees. The National Ag Center has contacts from coast to coast, and manages to stay involved on many levels through work with groups like the Natural Resources Conservation Service (NRCS), Cooperative State Research, Education, and Extension Service (CSREES), Sustainable Agriculture Research and Education program (SARE), Appropriate Technology Transfer for Rural Areas (ATTRA), and even the United States Customs Office.
Partnerships with academic groups keep the National Ag Center up to date on new trends. The National Ag Center is working with the University of Wisconsin to develop Environmental Management Systems (EMS) for livestock operations. The National Ag Center also partners with the USDA’s Pest Management Centers, in particular the North Central Pest Management Center, which are promoting sound pest management techniques.
Employees from the National Ag Center spend time in the field as well. They visit farm shows and growers’ conferences; they take the initiative to get out and meet the farmers. For the past several years, Missouri Farm Bureau has invited the National Ag Center to present their materials at the Western States Farm Show, providing an additional opportunity to get to know its clientele. Direct contact with farmers gives the National Ag Center a perspective unique within the EPA, allowing it to better serve the ag community’s needs.
Agricultural compliance is a multifaceted problem, so the National Ag Center approaches the problem from many angles thanks to its interactions with the national ag community. Working closely with the ag community from coast to coast helps ensure that everyone’s needs are addressed.
What Is the National Ag Center Doing to Help You?
The Center provides many products and services for the agricultural community:
- A regularly updated Web site (http://www.epa.gov/agriculture) with current news and user-friendly materials consolidating information about compliance requirements, pollution prevention, and technical assistance resources for use by farmers, regional and state assistance and educational programs, trade associations, businesses, and local governments. The Web site also provides links to information sources outside the EPA.
- An electronic news service for periodic updates on the new information in the National Ag Center’s Web site.
- A toll-free number (with real people 9-5 CST!) for those with questions on agriculture compliance issues. The National Ag Center has handled questions dealing with everything from ostrich carcasses to bat guano, and they are eager to take your questions.
- Numerous EPA documents that may be downloaded from the site or requested in hard copy from the National Ag Center. These materials are available for individual use or in bulk for use in training sessions.
What’s New At the National Ag Center?
Livestock and Poultry Environmental Stewardship (LPES) Curriculum
The National Ag Center partnered with the U.S. Department of Agriculture (USDA) to fund the development of LPES, a national education program applying the principles of environmental stewardship to livestock and poultry production. The curriculum, a collaborative effort of fifteen land-grant universities, MidWest Plan Service (MWPS), the National Ag Center, and the USDA, focuses on total nutrient management strategies and land stewardship, and provides environmental management evaluation tools for farmers and ranchers. The curriculum materials are available free online (http://www.lpes.org) or can be ordered in hardcopy or CD format at http://www.mwpdhq.org.
Worker Protection Standard (WPS) Fact Sheets
The WPS works to reduce pesticide-related illness and injury for pesticide workers, handlers, and employers. The National Ag Center developed a series of fact sheets addressing the most common questions it receives concerning the WPS. Topics include posting requirements, safety equipment, and information exchange. The fact sheets are available via the National Ag Center homepage (http://www.epa.gov/agriculture) under Publications, or by calling 1-888-663-2155 toll-free.
Animal Agriculture Fact Sheets
The National Ag Center has produced a fact sheet and brochure entitled, “What to Expect When EPA Inspects Your Livestock Operation”, designed to give livestock operation owners/managers an understanding of the EPA inspection process and possible results. Also available are a series of fact sheets regarding Concentrated Animal Feeding Operation (CAFO) compliance requirements. Copies of the fact sheets are available from the sources listed above.
Contact the National Ag Center
The National Ag Center is a service for America’s agricultural community. To improve its service, the National Ag Center needs to hear from you. Please contact the National Ag Center with your environmental compliance questions, and they will do their best to help! Reach the National Ag Center at:
Telephone, toll-free: 1-888-663-2155
Online: http://www.epa.gov/agriculture
Email: agcenter@epa.gov
Return to Table of Contents for the May, 2002 issue
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information Center On-Line) Home Page
Announcements & Upcoming ConferencesState Organic Program Announces Clopyralid GuidelinesClopyralid is a long-lasting herbicide used to control broadleaf weeds. It passes through animals and the composting process with little breakdown. The fact that it doesn't break down presents a problem for compost and manure. Compost contaminated with clopyralid may harm certain types of broadleaf plants such as beans, peas, sunflowers, peppers, tomatoes, and potatoes.The WSDA Organic Food Program 2002 application packets stated that compost contaminated with clopyralid would be prohibited from use on organic farms. Sampling conducted over the last few months has indicated that the majority of compost and manure has measurable amounts of clopyralid. The zero tolerance on clopyralid contamination has led to extremely limited sources of compost and manure that meet organic standards even though for many crops clopyralid-contaminated compost and manure has no detrimental effect on the plants or soil.The WSDA Organic Advisory Board approved the following recommendation that the WSDA Organic Food Program will adopt for the 2002 cropping season:
There will be no restrictions on organic growers utilizing compost or manure that is contaminated with clopyralid.Submitted to AENews by David Granatstein, Sustainable Agriculture Specialist, Center for Sustaining Agriculture and Natural Resources, Washington State University, 1100 Western Ave. N., Wenatchee, WA 98801. Tel. 509-663-8181 x.222, FAX 509-662-8714, email: granats@wsu.edu; Web site: http://organic.tfrec.wsu.edu/OrganicIFP/Home/Index.html. Food Safety Farm to Table Conference May 29-30The Food Safety Farm to Table conference will be held May 29 and 30 at the University Inn in Moscow, Idaho. The sessions will cover:
|
Return to Table of Contents for the May, 2002 issue
Return to Agrichemical and Environmental News Index
Return to PICOL (Pesticide Information
Center On-Line) Home Page



















